Research Article
Biomarkers of Inflammatory Cytokines & Adipokines Demonstrate the Interacting Influence in Patients with Metabolic Syndrome and Onset of Type 2 Diabetes in Indian Adults
901
Views & Citations10
Likes & Shares
Aim & objective: The aim of the current study was to analyze significantly Elevated Pro-inflammatory cytokines those play an emerging role in the metabolic syndrome patients with diabetes mellitus. The production of inflammatory IL-6, IL-17, IL-23& adipokines seem to be strong association in Patients with Metabolic Syndrome and Onset of Type 2 diabetes. These key profiling may form a target for novel treatment modalities with improving diagnostic approaches.
Methods: This case control study investigated 90 cases with Met-S diagnosed by IDF criteria and on-set of type 2 DM (male aged 36.92 ± 2.40 years); (female aged 36.76 ± 2.30 years) comprising 90 healthy control (male aged 37.28 ± 2.05 years); (female aged 35.36 ± 2.74 years) respectively. We compared the circulating levels of the serum cytokines IL-6, IL-23, IL-17 inflammatory biomarker and adipokines levels were investigated using by the ELISA method in Met-S with onset of Type 2 DM with all clinical settings.
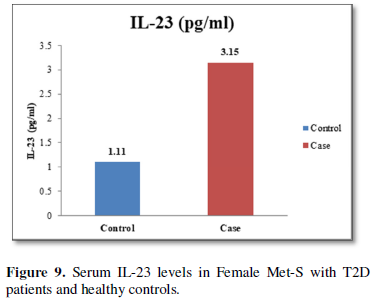
Results: The Serum levels of inflammatory cytokines significantly different IL-6, IL-17 and IL-23 were found in Met-S cases with type 2 diabetic patients than in healthy male & female subjects respectively (IL-6; 27.53 ± 2.61 vs. 6.07 ± 1.76 pg/ml male &24.15 ± 2.26 vs. 6.78 ± 1.37 pg/ml female;𝑃<0.001); (IL-17; 9.21 ± 0.55 vs. 2.05 ± 0.78 pg/ml male &7.64 ± 1.90 vs. 1.92 ± 0.50 pg/ml female; 𝑃<0.001); (IL-23; 3.64 ± 0.34 vs. 1.18 ± 0.02 pg/ml male &3.15 ± 0.48 vs. 1.11 ± 0.14 pg/ml female; 𝑃<0.001). Additionally, the serum of adiponectin was found significant decreased and serum level of leptin were found significantly higher in patients with Met-S and type 2 DM than in healthy subjects (𝑃< 0.001).
Conclusions and Interpretation: Metabolic syndrome with onset Type 2 Diabetes had significantly greater cytokines level of serum IL-6, IL-17 and IL-23 than controls. The complications related to Met-S were key drivers of proinflammatory cytokine production and supporting the evidence that inflammation plays vital role in the immune-pathogenesis of the disease.
Additionally, this study clearly demonstrates that the circulating serum levels of proinflammatory cytokines and adipokines definitely have strong association in primary mediators of inflammation in Met-Scases with type 2 DM. Hence there is critical need for prevent the ongoing epidemic of CVD and diabetes mellitus for public health. Furthermore, research is encouraged to establish the efficacy of applying these inflammatory biomarkers to diagnosis and treatment in clinical setting.
Keywords: Inflammation, Adipokines, Proinflammatory Cytokines, Onset T2DM, Metabolic syndrome
Abbreviations: Met-S: Metabolic Syndrome; BMI: Body Mass Index; WC: Waist Circumference; FBS: Fasting Blood Sugar; HbA1c: Glycosylated Hemoglobin; CVD: Cardiovascular disease; TC: Total Cholesterol; TG: Triglyceride; LDL: Low Density Lipoprotein Cholesterol; HDL: High Density Lipoprotein-Cholesterol; VLDL: Very Low-Density Lipoprotein; Hs-CRP: High Sensitivity C-Reactive Protein; T2DM: Type 2 Diabetes Mellitus; NEFA: Non-Esterified Fatty Acids; IL: Interleukin.
INTRODUCTION
The prevalence of the metabolic syndrome is increasing in developed and developing countries [1]. Metabolic syndrome, alternatively known as insulin resistance syndrome or syndrome x, is a set of metabolic disorders that increase patients risks for cardiovascular disease and type 2 diabetes mellitus. The main clinical symptoms of Met-S include central obesity, hypertension, hyperglycemia, low high-density lipoprotein levels, and high triglycerides [2]. Metabolic syndrome is defined by the International Diabetes.
Federation as a waist circumference greater than 94 inches in Caucasian men and greater than 80 inches in Caucasian women plus at least two of the following risk factors: triglycerides greater than 150 mg/dl or taking lipid-lowering agents, HDL levels less than 40 mg/dl and 50 mg/dl in men and women, respectively, a systolic blood pressure above 130 mmHg or a diastolic blood pressure above 85 mmHg or taking medicine for high blood pressure, and a fasting blood sugar above 100 mg/dl or having T2DM [3].
Met-S is associated with an up to five-time increased risk of T2DMand an approximately two-fold increased risk of CVD [4]. This is due to clustering of various risk factors in patients with Met-S. Population-based cohort studies on subjects with and without CVD and diabetes mellitus (DM) at presentation have demonstrated that the mortality rates due to these conditions were greater in those with Met-S [5]. It is well accepted that the metabolic syndrome increases the risk for the development of cardiovascular disease, type 2 diabetes, stroke and cancer [2].
Inflammation is defined as the local physiological response to tissue injury and regulated by pro-inflammatory cytokines. Recently, chronic inflammation has received considerable attention as a very important pathophysiological mechanism in type 2 diabetes mellitus. Inflammatory cytokines have been postulated to be important pathogenic factors in the development of type 2 DM [6].
The link between inflammation, obesity and type 2 diabetes (T2D) is currently well established. Research from Hotamisligil [7] in the early 1990s pointed to a pathogenic role of inflammatory cytokines in the development of insulin resistance and diabetes. The authors showed that TNF-α level was proportional to insulin resistance in obese people and animals [7]. Further research, confirmed this hypothesis, demonstrating that TNF-α was capable to induce insulin resistance in lean animals [7-10]. Nowadays, it is known that several pro-inflammatory cytokines are capable to inhibit the insulin signaling pathway through the activation of molecular pathways such as Nuclear Factor Kappa-light-chain-enhancer of activated B cell (NF-κB), IB kinase-β (IKKβ) and Jun kinase (JNK) [11-15].
Pro-inflammatory cytokines and adipokines are raised due to the activation of macrophages and adipocytes in obese adipose tissue, which leads to a so called “chronically inflamed adipose tissue”. A disturbed lipid metabolism in overweight/obesity is also capable of inducing a chronic pro-inflammatory state [16,17]. In monocytes and macrophages, the Nlrp3 inflamma some senses lipotoxicity-associated increases in intracellular ceramide, there by inducing caspase-1cleavage and facilitating interleukin-1β (IL-1β) secretion [18]. Addition- ally, lipid alterations like high levels of Ox-LDL and low levels of HDL are related to inflammatory activation [19-21] while free fatty acids enhance these creation of T NF-α, IL-6 and PAI-1 from macrophages. Activated macrophages secrete ever more inflammatory cytokines and chemokines creating a feed-forward loop of inflammation [20,22]. In sum, the literature contains numerous reports on increased levels of pro-inflammatory cytokines in the metabolic syndrome (Met-S) and T2D, including excellent reviews on this topic [23-28].
In another recently published study, we reported on the serum levels of a panel of Met-S and T2DM-related cytokines, chemokines and growth factors, namely IL-8, TNFα, IL-6, IL-1β, CCL4, CCL2, Resistin, Leptin, Adiponectin, hepatocyte growth factor and PAI-1 [29].
Interleukin-6 is a pleiotropic cytokine with several functions in different tissues. Initially, interleukin-6 was described as an important factor of the immune system. However, it has been shown that this cytokine also plays a vital role in glucose homeostasis, especially on metabolic regulation [30].
Interleukin-17 is a newly identified inflammatory cytokine produced by activated and memory T lymphocytes. It has pleiotropic activities including the induction of diverse inflammatory cytokines (e.g., TNF-α and IL-6) and chemokines (e.g., CCL2/MCP- 1, CXCL1/KC and CXCL2/MIP-2) from a large variety of cells [31] Interleukin-17 acts as highly potent inflammatory cytokine that initiates tissue inflammation and induces the infiltration of other inflammatory cells into the target organs [32].
Increasing evidence suggests that interleukin-17 plays a crucial role in various inflammatory responses and autoimmune diseases [33]. Recent studies have shown that serum elevated Interleukin-17 levels were analyzed in STZ-induced diabetic animal models and non-obese diabetic mice from insulitis to diabetes [34,35]. However, to date, there are little published data evaluating the role of IL-17 in type 2 DM.
OBJECTIVES
The aim of the current study was to analyze significantly Elevated Pro-inflammatory cytokines those play an emerging role in the metabolic syndrome patients with diabetes mellitus. The production of inflammatory IL-6, IL-17, IL-23 & adipokines seem to be strong association in Patients with Metabolic Syndrome and Onset of Type 2 diabetes. The study was to clarify whether serum levels of IL-17 demonstrate a change in metabolic syndrome patients with type 2 diabetes, we compared its concentration in the serum IL-17 in 25 patients with type 2 diabetes and age-matched healthy controls. Serum concentrations of Ienterleukin-6 and Ienterleukin-23 were also measured in the same group because development and maintenance of Th17 cells, as the main source of Ienterleukin-17, require these two cytokines. Meanwhile, we evaluate the correlation between serum levels of IL-6, IL-17 and IL-23 with adipokines and anthropometric measurements involved in metabolic syndrome patients with onset of type 2diabetes. Our results suggest that IL-17 might be involved in the pathogenesis of type 2 diabetes as an inflammation cytokine.
RESEARCH DESIGN AND METHODS
Selection of Met-S patients and controls
A total of 180 subjects, including 45 healthy male & 45 healthy female both as controls from the Medicine Department at S.P. Medical College and associated group of P.B.M. Hospitals and 45 males as well as 45 females in metabolic syndrome patients with type 2 diabetes, were enrolled in the study. The clinical history and disease status of each participant were taken by a General Medicine. Metabolic syndrome was diagnosed based on the International Diabetes Federation (IDF) definition [36]. 90 patients with Met-S and onset of newly confirmed type 2 diabetes age between 25 to 40 years with Met-S diagnosed by IDF criteria and on-set of type 2 DM (male aged 36.92 ± 2.40 years); (female aged 36.76 ± 2.30 years) and exclusively HbA1c (%) level (Mean ± S.D.; 8.12 ± 0.96 male; 7.95 ± 0.82 female) were included in the study. The 90 healthy control subjects aged-matched individuals (male aged 37.28 ± 2.05 years); (female aged 35.36 ± 2.74 years) respectively were selected as controls healthy based on lack of prior medical history, lack of prescribed medication use, normal test results, also not suffering from any chronic illness and signed informed consent was procured by all the individuals before their inclusion in the study. We compared the circulating levels of the serum cytokines IL-6, IL-23, IL-17 inflammatory biomarker and adipokines levels were investigated using by the ELISA method in Met-S with onset of Type 2 DM with all clinical settings.
Ethics statement
This study involving humans was approved by the Developmental Research Committee (DRC) at the Rajasthan University of Health Sciences, Jaipur, India. The patients involved in this study had signed an informed consent prior to the start of this study.
Anthropometric measurement
At the time of presentation demographic details were noted down and complete history was obtained regarding the patients with metabolic syndrome, type 2 diabetes, hypertension, any inflammatory disease, joint pains, infections, hyperlipidemias, endocrine disorders and smoking habits. Drug history was also obtained. The details were noted on a questionnaire and the patient’s consent was also taken before initialization of the study.
Measurements of adiposity
Body Mass Index was calculated according to the formula as the ratio of weight (kg) to the square of height (m) (kg/m2). Waist circumference was measured at the slimmest point using a flexible tape with average of two measurements taken after subject inspiration and after expiration (mean between the two measurements @1.5cm) at the midpoint between the lowest rib and the iliac crest. Waist-hip ratio (WHR) defined as the ratio of waist girth to the circumference of the hips measured at the trochanter [36].
BIOCHEMICAL ANALYSIS
Blood sampling
Participants were asked to fast for 12 h before blood sampling. Venous blood samples were collected in sampling tube with aseptic precautions. After 2 h of collections sample was centrifuged at 3000 rpm for 5 min. Serum was separated and collected in polythene tube with cork. The sera with no sign of hemolysis used for the estimation of all biochemical parameters.
BIOCHEMICAL ESTIMATION
Insulin resistance markers
HbA1c concentration is measured based on a specific chemical reaction to the glycated N-terminal valine of the β-chain using by Quo-Lab A1C Analyzer. Fasting blood glucose was measured after overnight fasting by Glucose Oxidase-Peroxidase Method using BECKMAN COULTER Analyzer Model AU680.
Lipid parameters analysis
Serum Total Cholesterol was determined by the enzymatic colorimetric cholesterol oxidase-peroxidase method, Serum High Density Lipoprotein was estimated by the cholesterol esterase-peroxidase precipitation method and Serum Triglycerides was estimated by the GPO-PAP-enzymatic colorimetric method. All these parameters optical density (absorbance) was done on BECKMAN COULTER Analyzer Model AU680. Serum level of Low-Density Lipoprotein Cholesterol levels were calculated by Fried Wald equation.
Adiponectin analysis
Serum level of adiponectin was measured using ALPCO, USA, High Molecular Weight (HMW) & Serum Adiponectin ELISA kit for research use only.
Leptin analysis
Serum level of Leptin was estimated using by Diagnostic Automation, Inc., USA, Micro-well ELISA kit for research use only.
Free fatty acid analysis
Serum level of Free Fatty Acid was estimated using SIGMA ALDRICH, USA, Free Fatty Acid Quantitation kit, ELISA kit for research use only.
Cytokine ELISA assay
The levels of interleukin-17A and interleukin-23 in the serum were measured using an Enzyme Linked Immunosorbent Assay via Human cytokine ELISA set (R&D Systems, USA) and serum levels of IL-6, IL-17 and IL-23 were measured using kits from The Bio-Plex Pro Human Cytikine Assay, Bio-Rad Laboratories In. USA. The serum was isolated and stored at -80ºC until further analysis. ELISA was performed according to the manufacturer’s protocol. The absorbance was read at 450 nm with a microtiter plate reader (Thermo Scientific-MULTISKAN FC). Cytokine levels were determined with the help of standard curve and concentration was expressed as pg/ml.
Statistical data analysis
All numerical data were presented in terms of mean ± SD. Statistical analysis of results was completed by normal distribution ‘Z’ test. In this data analysis, variables showing p-value less than 0.05 and 0.001 were considered to be significant statistically as well as highly significant respectively 0.001. Correlation coefficient (r value) was calculated for final finding correlation between two parameters by using Pearson two-tailed analysis. All statistics were done using SPSS software version 15.
RESULTS
Clinical data of Met-S with T2DM patients
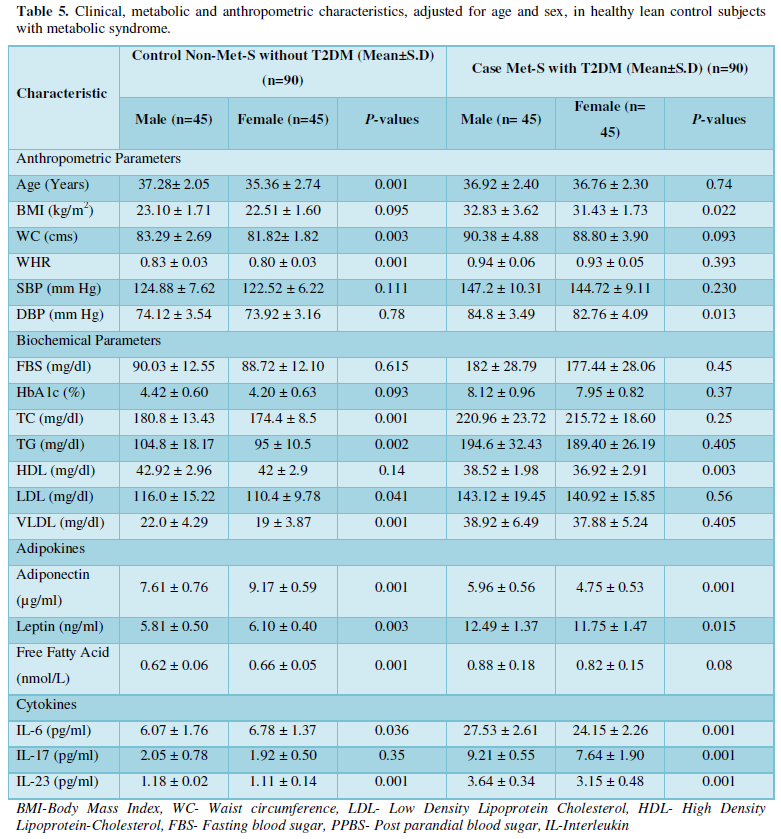
The clinical and demographic data of metabolic syndrome patients with type 2 diabetes were shown in Tables 1 and 2. Age and sex distributions were similar between patients and control subjects. Among the 45 recruited patients, men another 45 recruited patients were women. The average age of the patients was 36.92 ± 2.40 years. Healthy controls were 45 subjects with mean age 37.28 ± 2.05 years, male subjects and the average age of the patients was 36.76 ± 2.30 years. Healthy controls were 45 subjects with mean age 35.36 ± 2.74 years, female subjects. Exclusively, metabolic syndrome patients with onset of type 2 diabetes were involved by markedly higher levels of fasting blood glucose (FBG), glycosylated hemoglobin (HbAlc) along with anthropometric parameter under IDF definition criteria [36] compared to control subjects.

Determination of anthropometric parameters with serum level of Adipokines
Tables 1 and 2 shows the anthropometric measurements of the patients with metabolic syndrome (N=45) as cases and subjects without metabolic syndrome as healthy control (N=45). As expected, mean anthropometric parameters of BMI, Waist Circumference, Waist to Hip Ratio, Systolic blood pressure, Diastolic blood pressure were showed highly statistically significantly increased (P<0.001) in both the groups.
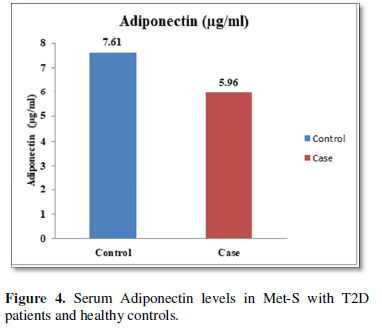
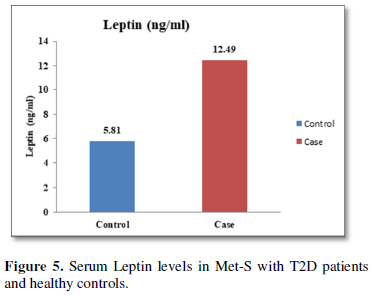
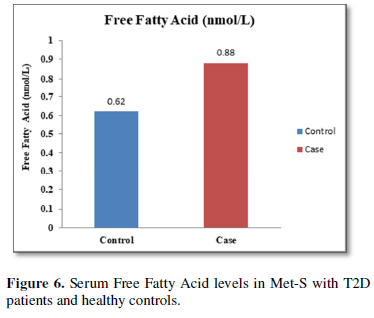
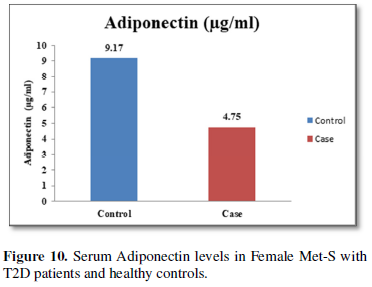
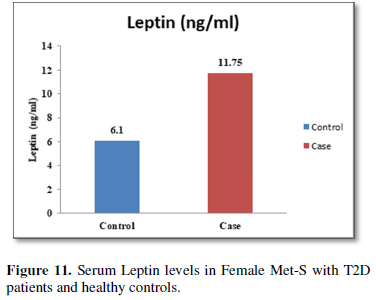
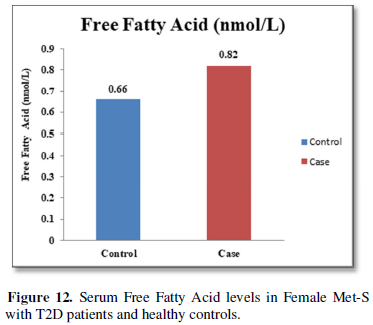
Additionally, biochemical parameters of the participants in the present study are given in the Tables 1 and 2. Serum Fasting Glucose, HbAlc, Triglycerides, Total Cholesterol, LDL-C, VLDL-C, Leptin and Free Fatty Acid levels were significantly increased (P<0.001) and HDL-C, adiponectin levels were significantly reduced (P<0.001) patients with Met-S and healthy compared to controls (Tables 1 and 2 and Figures 1-3).
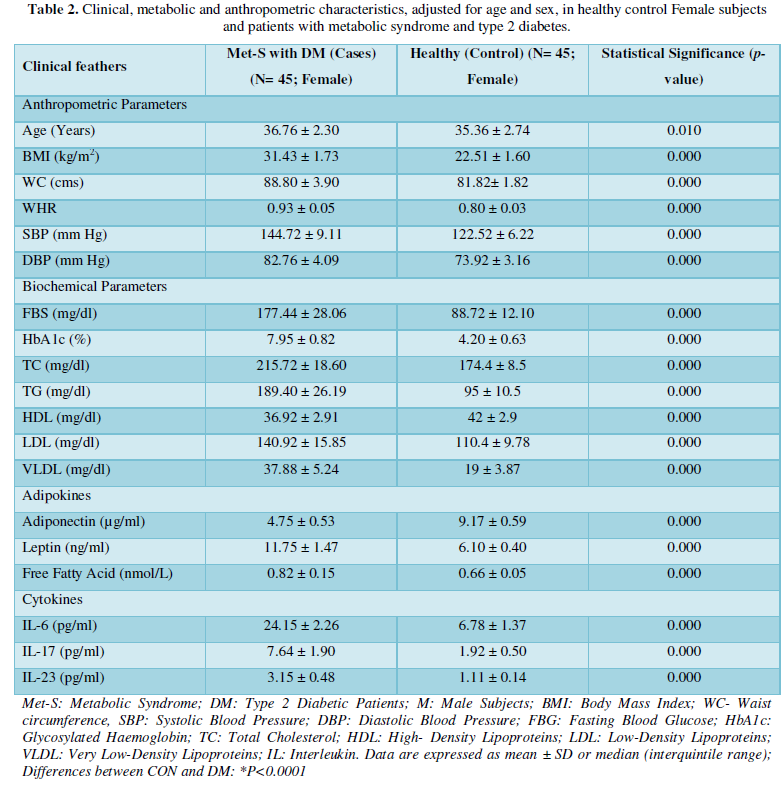






Determination of serum level of Th1 specific pro-inflammatory cytokines IL-6 and Th17 specific pro-inflammatory cytokines IL-17and IL-23
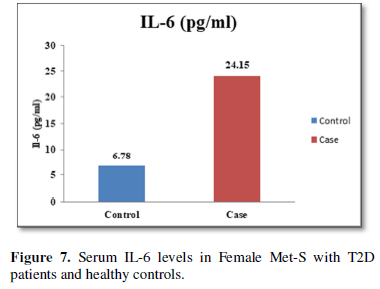
Table 1 and Figure 4 show that IL-6 level in metabolic syndrome patients with type 2 diabetes was tremendously higher than their respective healthy control subjects in males (p<0.001). There was a significant increase in serum IL-17 concentration in Met-S with onset of T2D aged below 40 years male as compared to controls and the age associated augmentation in IL-17 levels was also highly significant (p<0.001) (Figure 5). A similar increasing pattern was found in the serum levels of IL-6, another Th1 specific pro-inflammatory cytokine (Tables 1 and 2 and Figure 7).
The increased level of pro-inflammatory cytokines suggests that innate immune system also plays a crucial role in the pathogenesis of type 2 diabetes. So, it might be indicating that the IL-17 related cytokines may also be involved in the pathogenesis of type 2 diabetes. Furthermore, the level of IL-23 was found to be significantly increased in Met-S with type 2 DM patients of age groups as compared to healthy controls (p<0.001) (Table 1 and Figure 6). Statistical analysis showed a significant difference between serum levels of IL-6, IL-17 and IL-in metabolic syndrome patients with type 2 diabetes and healthy controls (𝑃<0.001) (Tables 1 and 2 and Figures 4-12).
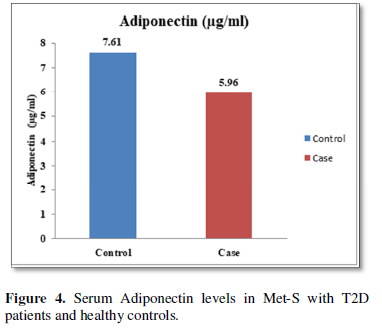

Determination of serum level of Th1 specific pro-inflammatory cytokines IL-6 and Th17 specific pro-inflammatory cytokines IL-17and IL-23
Table 1 and Figure 4 show that IL-6 level in metabolic syndrome patients with type 2 diabetes was tremendously higher than their respective healthy control subjects in males (p<0.001). There was a significant increase in serum IL-17 concentration in Met-S with onset of T2D aged below 40 years male as compared to controls and the age associated augmentation in IL-17 levels was also highly significant (p<0.001) (Figure 5). A similar increasing pattern was found in the serum levels of IL-6, another Th1 specific pro-inflammatory cytokine (Tables 1 and 2 and Figure 7).
The increased level of pro-inflammatory cytokines suggests that innate immune system also plays a crucial role in the pathogenesis of type 2 diabetes. So, it might be indicating that the IL-17 related cytokines may also be involved in the pathogenesis of type 2 diabetes. Furthermore, the level of IL-23 was found to be significantly increased in Met-S with type 2 DM patients of age groups as compared to healthy controls (p<0.001) (Table 1 and Figure 6). Statistical analysis showed a significant difference between serum levels of IL-6, IL-17 and IL-in metabolic syndrome patients with type 2 diabetes and healthy controls (𝑃<0.001) (Tables 1 and 2 and Figures 4-12).

Baseline correlation between anthropometric variables and markers of Met-S with T2DM Cases
BMI and WC were correlated significantly with age, BMI, WC, WHR, SBP, DBP, fasting blood sugar, HbA1c, Total Cholesterol, Triglycerides, HDL, LDL, VLDL, Adiponectin, Leptin and Free fatty acid with respectively (BMI; WC) all correlation were found to be statistically significant at the 0.01 level (Table 2-5).









DISCUSSION
This study shows that there are considerable differences in clinical profiles between inflammatory biomarkers such as IL-6, IL-17, IL-23 and adipokines in metabolic syndrome patients with type 2 diabetes male & female adults in Indian.
Metabolic syndrome is defined by constellation of inter-connected with clinical, biochemical and metabolic factors that strongly increases the risk of cardiovascular disease, type 2 diabetes and all causes of mortality [37,38]. The metabolic syndrome represents a combination of risk factors, which include atherogenic dyslipidemia, hypertension, hyperglycemia, prothrombotic state and a pro-inflammatory state [39].
The relationships between inflammatory biomarkers such as IL-6, IL-17, IL-23 and adipokines in metabolic syndrome patients with type 2 diabetes have not been thoroughly investigated. This study was designed to investigate that the circulating levels of serum IL-6, IL-17, IL-23 and adipokines in metabolic syndrome with onset of type 2 diabetes mellitus. First, we have found that significantly elevated in the circulating serum levels of pro-inflammatory cytokines IL-6, IL-17, IL-23 in Met-S patients with type 2 diabetes were compared to healthy subjects. Second, we have attempted to establish a relationship of between age and sex with anthropometric and serum adiponectin, leptin, HbA1c, fasting blood sugar, lipid profile levels of metabolic syndrome cases with onset of Type 2 diabetes mellitus were investigated. We observed significant decrease Adiponectin level. While, serum level of leptin, fasting blood sugar, waist circumference and BMI with all anthropometric feathers were significantly higher in metabolic syndrome patients with type 2 diabetes than in the controls. Moreover, BMI and WC significantly correlate with anthropometric parameter, adiponectin, leptin, free fatty acid, HbA1c, fasting blood sugar, lipid profile levels. These results suggested that serum IL-6, IL-17, IL-23 and adipokines might participate in the inflammatory process of type 2 diabetes and have a crucial role in the pathogenesis of in Met-S with onset of type 2 diabetes mellitus.
The relation between IL-6 and inflammation-induced obesity in type 2 diabetic patients
Over the past decades many studies have suggested that low-grade inflammation related to central obesity might be the key regulator in pathogenesis of type 2 diabetes mellitus [40]. It has been confirmed that enlargement of adipose tissue is associated with increases of number of adipose tissue macrophages, which are responsible for increases in serum concentration of pro-inflammatory cytokines, especially IL-6 and TNF-α expression. IL-6 is released from macrophages of adipose tissue as well as from adipocytes and skeletal muscle [41,42].
In addition, previous studies revealed an association between IL-6 and systemic inflammation causing Met-S [43]. This study showed that serum IL-6 levels were significantly greater in Met-S patients than in controls. No significant correlations between serum IL-6 levels and Met-S components were observed. Previous studies showed that IL-6 is positively associated with BMI, fasting insulin, hypertension and type 2 diabetes; however, such results disagree with our findings Sarbijani [45] reported that IL-6 serum levels were significantly greater in men with Met-S than in controls. They also observed a lack of correlation between IL-6 and Met-S components, which agrees with our findings. Additionally, Kitsios [46] showed that obese and overweight adolescents and children with Met-S had significantly greater serum IL-6 levels than their counterparts without Met-S.
Moreover, recent study Mojgan [47] reported that serum IL-6 and TNF-α levels were significantly greater in metabolic syndrome patients with type 2 diabetes than in controls, supporting the evidence that inflammation plays an important role in the immune-pathogenesis of the disease. However, no correlation was found between Met-S components and IL-6 or TNF-α serum level. Rehman and Victoria [48,49] studies were observed similarly serum IL-6 were significantly higher in the diabetic patients than in the related controls. These all studies supported by our study results and these data support a possible role for inflammation in diabetogenesis. Thus, our results have confirmed these findings in metabolic syndrome patients with type 2 diabetes had significantly increased serum levels of IL-6 with other pro-inflammatory cytokines than healthy controls. These results support the evidence that inflammation plays an important role in the immune-pathogenesis of the disease. Additionally, we suggest that IL-6 and other pro-inflammatory cytokines serum levels be measured as valuable predicting factors for Met-S.
The relation between IL-17, IL-23 and inflammation-induced obesity in patients with type 2 diabetes
Our data indicated that serum IL-17, IL-23 levels were higher in metabolic syndrome patients with type 2 diabetes group in comparison with the healthy control group. Recent studies indicated that IL-17 has a crucial role in the creation of inflammation in adipose tissues of obese individuals [50]. In animal model, obesity has been correlated with elevated levels of IL-17 and increased inflammation [50]. Importantly, Jagannathan [51] concluded that patients with type 2 diabetes showed increased Th17 cells with activated Th17 signature genes. Obese patients who have insulin resistance showed elevated blood levels of IL-17. Moreover, it has been demonstrated that circulating levels of IL-17 are elevated significantly in patients with diabetes [51] and obese women [52]. Therefore, both IL-17and IL-23 are introduced as additional markers for inflammation that accompanies obesity. In addition, earlier findings confirmed the increase in leptin and macrophage migration inhibitory factor levels in obese humans [53].
Role of IL-17 in type 2 diabetes mellitus development
Recent studies have demonstrated an association between Th17 cells and T2D development. Several investigators have suggested that T2D and its complications are immune-dependent conditions that can alter patterns of cytokines expression [53]. Chen [54] reported that IL-17 levels are elevated in newly diagnosed Type 2 diabetic patients than control subjects. Moreover, they found an increase in IL-17 mRNA expression and ROR γt in peripheral blood mononuclear cells (PBMCs) of type 2 diabetic patients and such increase in IL-17 gene expression were associated with TNF-α gene expression. Such findings indicate that IL-17 plays a crucial role in the inflammatory process and type 2 diabetes mellitus development. Additionally, treatment with anti-IL-17 neutralizing antibodies elevated serum adiponectin concentration, decreased serum levels of TNF-α and enhanced adipocyte-differentiation markers. These data indicate that IL-17 could possess a crucial role in development of insulin resistance and type 2 diabetes mellitus [55].
Several results concluded that dys-regulation of IL-17 production can result in excessive pro-inflammatory cytokines expression and chronic inflammation. Such inflammatory conditions may have a vital role in progression of insulin resistance [53]. Based on the above-mentioned findings, it can speculate that IL-17 involves in type 2 diabetes mellitus pathogenesis, however the exact mechanistic pathways are unclear yet. Previous studies suggested some probable mechanisms regarding the potential role of IL-17 in type 2 diabetes mellitus pathogenesis. Briefly, IL-17 activates nuclear factor-kappa B (NF-κB) pathway which up-regulates inflammatory cytokine genes expression [56].
IL-17A is a potential inflammatory cytokine which contributes to several autoimmune and inflammatory diseases including type 2 diabetes mellitus [57]. Several studies showed that Interleukin-17A could be considered as a potent inducer of type 2 diabetes mellitus. This present study revealed that the circulating levels of serum IL-6, IL-17, IL-23 and adipokines in metabolic syndrome with onset of type 2 diabetes mellitus. We have found that significantly elevated in the circulating serum levels of pro-inflammatory cytokines IL-6, IL-17, IL-23 in Met-S diagnosed patients with type 2 DM were compared to healthy subjects in Indian population.
Adipose tissue has been postulated to play a prominent role in both insulin resistance and the clinical expression of the metabolic syndrome, most likely mediated by increased release and peripheral tissue action of NEFA and by dysregulated production of adipocyte-secreted proteins, including leptin, adiponectin, resistin, TNF-α and IL-6 [57].
Our previous study, we observed the serum levels of adipokines, biochemical markers with anthropometric features were significantly higher in metabolic syndrome patients with type 2diabetes compare to healthy control subjects. These findings were also consistently similar with present study [58,59].
CONCLUSION
The current data of the present study suggest a strong association between advancement of Metabolic Syndrome with elevated level of pro-inflammatory cytokines as well as significant altered serum levels of adipokines. The results of the present study clearly demonstrate that the circulating serum levels of pro-inflammatory cytokines IL-6, IL-17 and IL-23 are primary mediators of inflammation in Met-S with Type 2 Diabetes. The role of Th17 cytokines also seems to be inevitable. IL-17 and IL-23 can be considered as a novel cytokine involved in the inflammatory process ensued in Met-S with Type 2 Diabetes.





Furthermore, cytokines produced by the Th17 path-ways might be implicated in clinical manifestation of diabetes and could be used as markers to distinguish between T1D and T2D at different age of diabetes onset. The results of this study indicate a close association between age and gender of T2D patients with adipokines and inflammatory markers specially IL-6, IL-23 and IL-17A. The study also suggests an age-related inflammatory change in T2D with Metabolic syndrome subjects as compared to healthy individuals. Additionally, to the best of our knowledge, the present work for the first time shows correlation between pro-inflammatory cytokines and adipokines along with metabolic anthropometric with age, BMI and WC in onset of Type 2 Diabetic human subjects having poor glycemic control with average HbA1c values above 8.2%. The cytokines viz. IL-6, IL-23, and IL-17A are believed to contribute to the pathogenesis of Type 2 Diabetes with Met-S on the basis of age and glycemic condition of the patients.
Furthermore, the adiponectin and leptin were significantly altered in patients with metabolic syndrome and type 2 diabetes. A significant a positive relationship between adipokines, free fatty acid and other parameters of metabolic syndrome, including BP values in normo-tensive subjects with central obesity in both male & female adults were also observed. Further, prospective in-depth studies are needed to validate this threshold and may provide new insights into the immunological and metabolic events which occur during Type 2 Diabetes with Met-S on. Furthermore, research and investigations are necessary to determine the efficacy of applying these biochemical markers to diagnosis and treatment in all clinical setting.
DISCLOSURE STATEMENT
This study is investigator initiated. The authors declare that they have no competing/conflict of interests in relation to this research work.
What’s New?
- Evidences of the emerging role of Interleukin-23, IL-17, IL-6 and adipocytokines play a pivotal role in the pathogenesis of onset Type 2 Diabetes with Metabolic syndrome both Sex.
- We found that the significant elevated serum levels of IL-6, IL-17 and IL-23 in onset of Type 2 Diabetes with association of different anthropometric measurements in Metabolic syndrome.
ACKNOWLEDGEMENT
This work was supported by Dr. R.P. Agarwal, Principal & Controller, Sardar Patel Medical College, Bikaner. Dr. Yogita Soni, Sr. Professor & Head, Guide & the study principal investigator. The authors are grateful to Professor for her helps in scientific discussion and English revision.
- Eckel RH, Grundy SM, Zimmet PZ (2005) The metabolic syndrome. Lancet 365: 1415-1428.
- Cheung BM, Wat NM, Man YB, Tam S, Cheng CH, et al. (2008) Relationship Between the Metabolic Syndrome and the Development of Hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study-2 (CRISPS2). Am J Hypertens 21: 17-22.
- International Diabetes Federation Press Conference (2005) The IDF consensus worldwide definition of the metabolic syndrome [article online], 2005. Available online at: https://www.pitt.edu/~super1/Metabolic/IDF1.pdf
- Grundy SM (2012) Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 59: 635-643.
- Ginsberg HN, MacCallum PR (2009) The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: Part I. Increased cardiovascular disease risk and the importance of atherogenic dyslipidemia in persons with the metabolic syndrome and type 2 diabetes mellitus. J Cardiometab Syndr 4: 113-119.
- Crook MA (2014) Type 2 diabetes mellitus: A disease of the innate immune system? An update. Diabet Med 21: 203-207.
- Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 259: 87-91.
- Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM (1996) Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 271: 13018-13022.
- Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 271: 665-668.
- Lin D, Shuyue W, Libo S, Xiuqing H, Yang Z, et al. (2017) Mir-338-3p Mediates Tnf-A-Induced Hepatic Insulin Resistance by Targeting PP4r1 to Regulate PP4 Expression. Cell Physiol Biochem 41: 2419-2431.
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, et al. (2006) Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw 17:4-12.
- Hooper PL, Hooper PL (2009) Inflammation, heat shock proteins, and type 2 Cell Stress Chaperones 14: 113-115.
- Israël A (2010) The IKK complex, a central regulator of NF-kappa B Cold Spring Harb Perspect Biol 2: a000158.
- Nakamori Y, Emoto M, Fukuda N, Taguchi A, Okuya S, et al. (2006) Myosin motor Myo1c and its receptor NEMO/IKK-gamma promote TNF-alpha-induced serine307 phosphorylation of IRS-1. J Cell Biol 173:665-671.
- Scazzocchio B, Varì R, D'Archivio M, Santangelo C, Filesi C, et al. (2009) Oxidized LDL impair adipocyte response to insulin by activating serine/threonine kinases. J Lipid Res 50: 832-845.
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, et al. (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821-1830.
- Sorisky A, Molgat ASD,Gagnon AM (2013) Macrophage-Induced Adipose Tissue Dysfunction and the Preadipocyte: Should I Stay (and Differentiate) or Should I Go? Adv Nutr 4: 67-75.
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, et al. (2011) The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med 17: 179-188.
- Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5: 150-159.
- Wilcox G (2005) Insulin and insulin resistance. Clin Biochem Rev 26: 19-39.
- Karki S, Chakrabarti P, Huang G, Wang H, Farmer SR, et al. (2011) The multi-level action of fatty acids on adiponectin production by fat cells. PLoS One 6: e28146.
- Hajer GR, Haeften TWN, Visseren FLJ (2008) Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J 29: 2959-
- Qu D, Liu J, Lau CW, Huang Y (2014) IL-6 in diabetes and cardiovascular complications. Br J Pharmacol 171: 3595-3603.
- Spranger J, Kroke A, Möhlig M, Hoffmann K, Bergmann MM, et al. (2003) Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes 52: 812-817.
- Pickup JC (2004) Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 27: 813-823.
- Febbraio MA (2014) Role of interleukins in obesity: Implications for metabolic Trends Endocrinol Metab 25: 312-319.
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE (2004) Inflammatory Markers and Risk of Developing Type 2 Diabetes in Diabetes 53: 693-700.
- Han JM, Levings MK (2013) Immune Regulation in Obesity-Associated Adipose J Immunol 191: 527-532.
- Baldeón RL, Weigelt K, Wit HD, Ozcan B, Oudenaren AV, et al. (2014) Decreased Serum Level of miR-146a as Sign of Chronic Inflammation in Type 2 Diabetic PLoS One 9: e115209.
- Pal M, Febbraio MA, Whitham M (2014) From cytokine to myokine: The emerging role of interleukin-6in metabolic regulation. Immunol Cell Biol 92: 331-339.
- Alessi MC, Juhan-Vague I (2006) PAI-1 and the metabolic syndrome: Links, causes and consequences. Arterioscler Thromb Vasc Biol 26: 2200-2207.
- Chen G, Liu C, Yao J, Jiang Q, Chen N, et al. (2010) Overweight, obesity and their associations with insulin resistance and cell function among Chinese: A cross-sectional study in China. Metabolism 59:1823-1832.
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel L, et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig 112: 1796-1808.
- Schmidt MI, Duncan BB, Sharrett AR, Lindberg G, Savage PJ, et al. (1999) Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities’ study): A cohort study. Lancet 353: 1649-1652.
- Popko K, Gorska E, Emmel AS, Plywaczewski R, Stoklosa A, et al. (2010) Pro-inflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res 15: 120-122.
- Alberti G, Zimmet P, Shaw J, Grundy SM (2005) The IDF consensus worldwide definition of the metabolic syndrome. Lancet 366: 1059-1062.
- Pal M, Febbraio MA, Whitham M (2014) From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol 92: 331-339.
- Chen G, Liu C, Yao J, Jiang Q, Chen N, et al. (2010) Overweight, obesity, and their associations with insulin resistance and cell function among Chinese: A cross-sectional study in China. Metabolism 59: 1823-1832.
- Alessi MC, Vague IJ (2006) PAI-1 and the metabolic syndrome: Links, causes, and consequences. Arterioscler Thromb Vasc Biol 26: 2200-2207.
- Bhatnagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, et al. (1995) Coronary risk factors in people from the Indian Subcontinent living in west London and their siblings in India. Lancet 345: 405-409.
- Weisberg SP, McCann M, Desai M, Rosenbaum R, Leibel L, et al. (2003) Obesity is associated with macrophage accumulation in adipose tissue. J Clin Investig 112: 1796-1808.
- Popko E, Gorska A, Emmel AS, Plywaczewski R, Stoklosa A, et al. (2010) Proinflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res 15: 120-122.
- Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP (2005) Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arteriosclerosis, Thrombosis, and Vascular Biology 25: 1268-1273.
- Lukic L, Lalic NM, Rajkovic N, Jotic A, Lalic K, et al. (2014) Hypertension in obese type 2 diabetes patients is associated with increases in insulin resistance and IL-6 cytokine levels: Potential targets for an efficient preventive intervention. Int J Env Res Pub He 11: 3586-3598.
- Sarbijani HM, Khoshnia M, Marjani A (2016) The association between Metabolic Syndrome and serum levels of lipid peroxidation and interleukin-6 in Gorgan. Diabetes Metab Syndr 10: S86-S89.
- Kitsios K, Papadopoulou M, Kosta K, Kadoglou N, Chatzidimitriou D, et al. (2012) Interleukin-6, Tumor Necrosis Factor alpha and Metabolic Disorders in Youth. J Clin Endocrinol Metab 2: 120-127.
- Mojgan M, Mohammad HG, Majid A, Mohammad RM, Mohammad MH (2017) Clinical Significance of Serum IL-6 and TNF-α Levels in Patients with Metabolic Syndrome. Rep Biochem Mol Biol 6: 76-79.
- Rehman K, Akash MSH, Liaqat A, Kamal S, Qadir MI, et al. (2017) Role of Interleukin-6 in Development of Insulin Resistance and Type 2 Diabetes Mellitus. Crit Rev Eukaryot Gene Expr 27: 229-236.
- Victoria L, Chanchal L, Shaini L, Abhishek D, Chubalemla L, et al. (2016) Interleukin-6 in obese type II diabetes with hypertension. Int J Res Med Sci 4: 896-901.
- Gonzalez FZ, Auguet T, Aragones G, Jurado EG, Berlanga A, et al. (2015) Interleukin-17A Gene expression in morbidly obese women. Int J Mol Sci 16: 17469-17481.
- Bogdan MJ, McDonnell ME, Shin H, Rehman Q, Hasturk H, et al. (2011) Elevated proinflammatory cytokine production by a skewed T cell compartment requires monocytes and promotes inflammation in type 2 diabetes. J Immunol 186: 1162-1172.
- Dumanovic MS, Stevanovic D, Ljubic A, Jorga J, Simic M, et al. (2009) Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women Int J Obes 33:151-156.
- Lago F, Dieguez C, Reino JG, Gualillo OV (2007) The emerging role of adipokines as mediators of inflammation and immune responses, Cytokine Growth Factor Rev 18: 313-325.
- Chen C, Shao Y, Wu X, Huang C, Lu W (2016) Elevated interleukin-17 levels in patients with newly diagnosed Type 2 diabetes mellitus. Biochem Physiol 5: 2.
- Ohshima K, Mogi M, Jing F, Iwanami J, Tsukuda K, et al. (2012) Roles of interleukin 17 in angiotensin II type 1 receptor-mediated insulin resistance. Hypertens 59: 493-499.
- Zepp J, Wu L, Li X (2011) IL-17 receptor signaling and T helper 17-mediated autoimmune demyelinating disease. Trends Immunol 32: 232-239.
- Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G (2001) Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 280: E745-E751.
- Ghanshyam G, Yogita S, Rajkumar V, Gajanand J (2018) Association of adipokines with metabolicsyndrome in North-West Rajasthan (India). Am J Biochem 8: 45-55.
- Gahlot G, Soni Y, Joshi G, Vyas RK, Agarwal RP (2020) Evidences for IL-6, IL-23, IL-17 and Adipokines in Patients with Metabolic Syndrome and Type 2 Diabetes Immunopathogenesis. Adv Nanomed Nanotechnol Res 2: 112-121.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Rheumatology Research (ISSN:2641-6999)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Allergy Research (ISSN:2642-326X)













