Research Article
Enhancing the Structure of Sandy Soil by Biological Molecules: An Innovative Approach to Water Conservation in Newly Reclaimed Desert-Agricultural Land
848
Views & Citations10
Likes & Shares
The main aim of this study was to enhance the water holding capacity of the reclaimed desert sandy soil using an environmentally friendly biological technique that would minimize the loss of irrigation water from plant root zone. by downward seepage. Two environmentally friendly bacteria (Azotobacter chroococcum and Lactobacillus fermentum) capable of producing insoluble polysaccharides as a soil pores plugging agent were selected. The polysaccharides production efficiencies of these bacteria were evaluated. The effectiveness of the polysaccharides in enhancing the water holding capacity of the soil was evaluated. The result showed that the sandy soil used in this study had a particle density of 2.58 g/cm3, a bulk density of 1.6 g/cm3and a porosity of 37.98 %. The particle size varied from 0.150 to 2.000 mm with most of the soil particles having a diameter within the range of 0.425-0.850 mm indicating that the soil was free of silt and clay. This soil had a loose texture, high infiltration rate and low water holding capacity. Azotobacter chroococcum and Lactobacillus fermentum were capable of producing levan from sucrose. The levan yield was 0.248 glevan/g sucrose (62.78% of theoretical yield) and 0.371 g levan/g sucrose (93.92% of theoretical yield) for Azotobacter chroococcum and Lactobacillus fermentum, respectively. The cell yield was 0.074 g cell/g sucrose (47.69 % of theoretical yield) and 0.062 g cell/g sucrose (56.92 % of theoretical yield) for Azotobacter chroococcum and Lactobacillus fermentum, respectively. The polymer was effective as a plugging agent to plug the pores of the high permeability sandy soil. The results showed that increasing the concentration of bacteria had no significant effect on the amount of leachates collect from the soils treated with both bacteria. However, the leachates collected from the soils treated with Azotobacter chroococcum were much larger than those collected from the soils treated with Lactobacillus fermentum. Also, the leachates collected from the control (soils received no bacterial treatment) were much larger than soils treated with both bacteria. These microorganisms were suitable for production of levan from sucrose, fixing nitrogen in soil and producing growth hormones, thereby improving the water holding capacity of the soil and enhancing its nutrient content and stimulating plant growth.
Keywords: Sandy soil, Particle density, Bulk density,Porosity, Water holding capacity,Infiltration, Seepage, Bio-cementation, Bio-logical sealing
INTRODUCTION
Egypt is a transcontinental country situated mostly in north-eastern Africa, with the Sinai Peninsula in Western Asia. Egypt has a coastline at the Mediterranean Sea in north, and the Gulf of Suez and the Red Sea in east. The country lies in the dry arid region except for the northern part which enjoys a Mediterranean climate during winter (December-March) which is cool, windy and humid, with occasional rains. Summer in Egypt (June-September) is very dry with extremely hot temperatures into the 32-38°C, sometimes breaking into 48°C. Shoulder seasons (April-May and October-November) are particularly pleasant months but with no rain. Egypt receives 20-200 mm of annual precipitation along the narrow Mediterranean Coast and nearly 0 mmin the central and the southern parts of the country [1, 2].
The total land area of Egypt is 1,000,450 km2, of which 32,425 km2(3.24%) is the Delta which is made of silt deposits carried by the River Nile and in which is most agricultural land and live most of the population. The Western Desert of Egypt (681,000 km2 or 68.07%) is an area of the Sahara which lies west of the River Nile up to the Libyan border, and from the Mediterranean Sea to the border with Sudan. The Eastern Desert (223,000 km2 or 22.29%) extends east from the Nile to the Red Sea, and from the Mediterranean Sea to the border with Sudan.The area of Sinai is 61,000 km2 (6.10%) and Northern Lakes cover an area of 3025 km2 (0.30%) and is main source of aquaculture in Egypt. Generally, desertsare barren areas of landscape (Figure 1) where little or no precipitation occurs and, consequently, living conditions are hostile for plant and animal life. With 96% of Egypt’s land is uninhabitable desert (never receives any rain) in both sides of the Nile, the population is concentrated around the narrow Nile Valley and Nile Delta, with smaller numbers along the Mediterranean and Red Sea coasts [3].
Egypt’s worrying population boom poses very real dangers to the economic development of the country and is considered as a major challenge to the government. In 2000, the United Nations estimated that Egypt’s population would hit 96 million in 2026. However, in 2017, there were some 104.5 million Egyptian, of which 9.5 million lived outside the country. With current population growth rate (2.6 million babies born in 2016)), Egypt’s population is expected to grow to 128 million by 2030 [4]. According to Egypt’s Statistical Agency, the population growth rate must be one-third that of economic growth to prevent living standards from deteriorating [5]. Once the breadbasket of the Roman Empire, Egypt began to import large quantities of wheat in the 1980sand is now importing 50% of its food [6].
The quest to bring desert land under cultivation has been a cornerstone of Egyptian Government Agricultural Policy since the 1952. The total area reclaimed reached 1.92 million feddans (feddan=0.42 hectare) in 1987. By 2002, the total reclaimable land was estimated at 2.8 million feddans [7]. However, the increase in agricultural land has not kept pace with the population increase in Egypt since 1950’s. As a result, the country is facing unprecedented challengesas the agricultural lands are increasingly strained due to urban expansionand depletion of scarce water resources as the Nile faces upstream challenges with Ethiopia building Africa’s largest dam [8]. Land reclamation in the Egyptian context means converting desert areas into agricultural land by extending water canals into the desert, enhancing soil fertility, and providing infrastructure for new village construction. If the unlimited desert sandy soil can be improved and provided with water, it can grow a lot of food for the growing population.
Therefore, in 2013, the Egyptian Government began an effort to reclaim approximately 1.5 million feddan of desert lands for agricultural use as a first stage of a major project aiming at the reclamation of 4 million feddan. Due to Nile water shortage, ground water will be used to irrigate 1,322,000 feddan (88.5%) and surface water will be used to irrigate 172,000 feddan(11.5%). The hope is that, with new wells, desalination plants and better infrastructure (new towns), farmers will be able to grow more wheat [2]. Example of this new development is the land reclamation project that began in 2015 in the Farâfra Depression (980km2) in Western Desert. The white desert of Farâfra(Figure 2) has been converted into agricultural land capable of producing wheat, potato, radish and other produces (Figure 3). By adding the new farmland to Egypt’s current 8.4 million Fedden, it is hoped to free the population from the narrow confines of the Nile Valley and have the capacity to meet food production needs [9].
Egypt desert soils originated by mechanical disintegration and wind deposit. Theyare mostly loamy sand (of 95-97% sand and 3-5% clay). These soils are coarse, porous and well-drained and have a red to brown color. They contain salts and are high in potassium, phosphorus and nitrates. These soils have very low moisture,very low organic matter and a basic pH (7.5-8.0). Generally, the sandy soils of Sahara are one of the poorest types of soil for growing plants because of their very low nutrients and very poor water holding capacity [10-14]. In addition, surface irrigation in this dry climate can cause the water to evaporate very quickly leaving salts behind on the soil surface causing salinization. Furthermore, water uses (agricultural, industrial, municipal, transportation and electricity generation) and management in Egypt are very complex and there is a great deficit between the demand and supply [15]. Therefore, it is important to consider (a) water conservation through use of new irrigation technology, (b) new water sources such as desalination and municipal wastewater treatment and reuse and (c) improving the quality of the soil and its water holding capacity [4,7,16].The farmer are already adopting new irrigation technology to conserve water [7] and the government has imparked on major desalination, and wastewater treatment and use projects as well as drilling wells for underground water [17]. However, building an adequate soil structure in the newly claimed land is still a major challenge.
In order to improve the soil properties, farmers are planting crops that fixes nitrogen such as alfalfa, but this process is unduly time-consuming for many farmers [4]. There are, however, several other techniques for land improvement including: (a) addition of biochar which significantly and permanently increase soil cation exchange capacity (the soil’s ability to hold nutrients), creates habitats for beneficial microbes and increases water retention [18-22], (b) addition of organic matter such as well-rotted manure or finished compost which decomposes quickly (since microbial activity is so fast in hot climate) and improves the physical properties of the soil [23,27], (c) application of chemical grouting to stabilize soil structure and modify the pore geometry of the soil by chemical reactions or ionic exchange resulting in a reduced fluid movement and improved water holding capacity and reduced water and nutrient seepage [28-32] and (d) application of microorganisms to alter the oil structure in order to reduce porosity and enhance water and nutrient retention [33-48].
In order to improve the soil properties, farmers are planting crops that fixes nitrogen such as alfalfa, but this process is unduly time-consuming for many farmers [4]. There are, however, several other techniques for land improvement including: (a) addition of biochar which significantly and permanently increase soil cation exchange capacity (the soil’s ability to hold nutrients), creates habitats for beneficial microbes and increases water retention [18-22], (b) addition of organic matter such as well-rotted manure or finished compost which decomposes quickly (since microbial activity is so fast in hot climate) and improves the physical properties of the soil [23,27], (c) application of chemical grouting to stabilize soil structure and modify the pore geometry of the soil by chemical reactions or ionic exchange resulting in a reduced fluid movement and improved water holding capacity and reduced water and nutrient seepage [28-32] and (d) application of microorganisms to alter the oil structure in order to reduce porosity and enhance water and nutrient retention [33-48].
There are many microbiological activities that can be used to alter soil structure and improve the properties of soils which include bio-cementation (or bio-mineralization), gleization and bio-sealing. Bio-cementation is the process where microorganisms produce elemental compounds such as calcium carbonate as a basis for bio-grout that can improve the mechanical properties of the soil and decrease its porosity [33-37]. Gleization is a process in which breakdown of soil structure takes place by strong oxidizing or reducing gelatinous agents which are the products of microbial metabolisms [38-41]. Soil bio-sealing is a process in which microbially induced compounds are utilized to plug the soil pores and reduce soil porosity, leading to increased water holding capacity and reduced loss of water and nutrient through seepage [42-48].
Natural bio-seal (biological soil crust) can develop from the intimate association between soil particles and microorganisms that live within soil such as cyanobacteria, green algae, fungi, bacteria, lichens and bryophytes [44]. They are typical of arid and semi-arid regions but can occur in most ecosystems [45-46]. Some strains of bacteria produce water insoluble polysaccharides which appear to be promising selective plugging agents that can be used to create bio-seal in the sandy soil of Egypt [49,50]. Microbial polysaccharides which have potential in the sealing mechanisms include dextran, xanthan, curdlan, indicant, pullulan, heteroglycan and zenflox-polysaccharides. This study proposes to investigate the possibility of applying biological sealing into the sandy soil of reclaimed Egyptian deserts and evaluate its effectiveness in improving water retention.
OBJECTIVES
The main aim of this study was to enhance the water holding capacity of the reclaimed desert sandy soil using environmentally friendly biological technique that will minimize the loss of irrigation water by downward seepage out of the plant root zone.The specific objectives were: (a) to select environmentally friendly bacteria capable of producing insoluble polysaccharides as a plugging agent in order to minimize soil porosity, (b) evaluate the polysaccharides production efficiency of these bacteria and establish the optimum concentrations of the bacterial cultures and (c) evaluate the effectiveness of the polysaccharides in enhancing the water holding capacity of the sandy soil.
MATERIALS AND METHODS
Selection of polysaccharide
The polysaccharide levan was selected for this study. Levan is a polymer made up of fructose (a monosaccharide sugar) connected in 2,6 beta glycosidic linkages as shown in Figure 4 [51,52]. Levan can be in both branched and linear structures (Figure 5) of relatively low molecular weight [52]. In the branched version, levan forms a very small, sphere-like structure. This structure has basal chains of 9 units long which contain 2,1 branching, allowing for the methyl ethers to form and create a spherical shape. The ends tend to contain a glucosyl residue. The branched structure of levan tends to be more stable than the linear structure. However, the amount of branching and length of polymerization tends to vary among different species. The shortest levan is 6-kestose, essentially a chain of two fructose molecules and a terminal glucose molecule [52-53].
Levan contains a diverse set of properties (Table 1). The beta 2,6 linkages of levan allow for it to be insoluble in water, oil and many organic solvents (methanol, ethanol, and isopropanol. The branching of levan also allow for it to have a large amount of tensile and cohesive strength, while the hydroxyl groups contribute to adhesion with other molecules [52-55].
Levan is diversity distributed in plants and microorganisms. It is usually found in the stems and leaf tissues of Agropyroncristatum, Dactylisglomerata, Pea secunda, Ttriticumaestivum and Pachysadra terminalis [56,57].
Levan is also produced as exopolysaccharides usually from sucrose (a disaccharide sugar containing glucose and fructose) based substrates by a variety of microorganisms including bacteria, fungi and algae. However, there are some reports indicating that microbial levan can be produced from fructose, glucose and raffinose substrates [56,58,59]. The main reaction in levan biosynthesis is the transfructosylation by the extracellular enzyme levansucrase. The enzyme forms the 2,1 linkages in the linear basal chains of levan to allow for branching points to occur. This production of levan is sensitive to temperature, oxygen concentration, pH and other factors [56,60-62].
Selection of microorganism
Selection of microorganism
Selection of microorganisms used in this study was based on the criteria shown in Table 2. As the land will be used for agriculture production, contaminated soil with pathogens could spread diseases to crops and vegetables or to healthy animals and human. Thus, the selected microorganisms must be non- pathogenic. Microbial cells smaller than the average pore sizes of the soil are desirable. Insoluble polysaccharide is required to plug the soil pores and form stable sealing. Arthrobacter and Bacillus are the most common bacterial genera found in soils and any microbes introduced into to the soil for the purpose of clogging the soil pores (bio-sealing) must compete with these indigenous bacteria for substrate [42,49,50].
Table 3 shows some of the levan producing bacteria. The bacterial species Azotobacter chroococcum and Lactobacillus fermentum were selected for this study. Azotobacter chroococcum are capable of producing levan and have a full range of enzymes needed to perform nitrogen fixation (ferredoxin, hydrogenase, and an important enzyme nitrogenase).Owing to their ability to fix molecular nitrogen and produce growth hormones, and therefore increase the soil fertility and stimulate plant growth, Azotobacter species are widely used in agriculture as a source of nitrogen biofertilizer [71]. Lactobacillus fermentum bacteria are a levan producing bacteria. The use of these two microorganisms would be suitable for production of levan from sucrose, fixing nitrogen in soil and producing growth hormones, thereby improving the water holding capacity of the soil and enhancing its nutrient content and stimulating plant growth. The scientific classifications of Azotobacter chroococcum and Lactobacillus fermentum are shown in Table 3 and their biological and biochemical characteristics areshown in Table 4.
Soil collection and preparation
The soil was collected from the Teaching and ExperimentalFarm of the Faculty of Agriculture, Cairo University. About 100 kg of soil were collected in plastic bags and transported to the Bioengineering Laboratory. The visible organic matter was removed from the soil and soil clumps were crushed.
A soil sample of 500g was used to determine the particle size distribution using a mechanical sieving apparatus (Vibratory Sieve Shaker, Series AS200, Retsch GMBH, Haan, Germany. The pan was first placed onto the sieving apparatus. The sieves with the smallest mesh were stacked on the top of the pan and successively larger meshes were placed above. The sample was placed into top sieve and the lid was placed on top of the stack. The shakerwasturned on for 30 min. The soil collected from each sieve was weighed and the percentage of each soil fraction from the original soil weight was calculated.
Soil samples of 50 g each were used to measure the soil particle density, bulk density and porosity. A soil sample of 50 g was placed in a 100 ml graduated cylinder and the actual volume of the soil sample was determined. Another soil sample of 50 g was placed into a graduated cylinder containing 100 ml of water. The volume of water that resulted from the addition of soil is considered the volume of the soil particles. The particle density is defined as the weight of the soil particles divided by their volume. The bulk density is defined as actual weight of the soil divided by its apparent volume. The soil porosity is defined as these were calculated as follows:
ρp = W/Vp(1)
ρb=W/Vb(2)
P=(ρb-ρp)/ρb(3)
where:
P = Porosity (%)
Vb= Volume of the soil(cm3)
Vp= Volume of the particles (cm3)
W = Weight of the soil (g)
ρb= Soil bulk density (g/cm3)
Vp=Particles density (g/cm3)
The rest of the soil was placed in 10 plastic bags, each containing 1 kg of soil. The bags placed in an autoclave (Tabletop Autoclave, Tuttnauer 2340M, Alpha Scientific, Vancouver, British Columbia, Canada) for sterilization at a temperature of 121°C and a pressure of 103 KPa for 20 min. This process was carried out to kill any soil microorganisms. The sterilized soil was used later to test the effectiveness of bio-cementation and bio-sealing (clogging of the soil pores).
The rest of the soil was placed in 10 plastic bags, each containing 1 kg of soil. The bags placed in an autoclave (Tabletop Autoclave, Tuttnauer 2340M, Alpha Scientific, Vancouver, British Columbia, Canada) for sterilization at a temperature of 121°C and a pressure of 103 KPa for 20 min. This process was carried out to kill any soil microorganisms. The sterilized soil was used later to test the effectiveness of bio-cementation and bio-sealing (clogging of the soil pores).
Preparation of the growth medium and microbial culture
Samples of Azotobacter chroococcum and Lactobacillus fermentum (Figure 6)were obtained from the Department of Microbiology, Faculty of Agriculture, Ein Shams University and the Department of Microbiology, Faculty of Agriculture, Cairo University, respectively.Liquid growth medium was prepared using Bacto® Nutrient Broth, which was obtained from Difco Laboratories, Detroit, Michigan, USA.
The Nutrient broth is composed of a simple peptone and a beef extract. The peptone contributes organic nitrogen in the form of amino acids and long-chained fatty acids while the beef extract provides vitamins, carbohydrates, salts and other organic nitrogen compounds.An amount of 6.5 g of the nutrient broth was added to two Erlenmeyer flasks, each containing 500 ml distilled deionized water. Each flask was capped and thoroughly mixed. The flasks were placed in an autoclave (Tabletop Autoclave, Tuttnauer 2340M, Alpha Scientific, Vancouver, British Columbia, Canada) at a temperature of 121°C and a pressure of 103 KPa for 20 minto sterilize the media. The flasks were left to cool down. One flask wasinoculated aseptically with Azotobacter chroococum while the other flask was inoculated aseptically with Lactobacillus fermentum. The two cultures were grown on a controlled environment laboratory shaker (MaxQTM 4000 Benchtop Orbital Shaker, Thermo Fisher Scientific, Montreal, Quebec, Canada) at room temperature (21°C) for 24 h.
The cell number was determined according to the procedure described by Ghaly and Mahmoud [217].
Culture propagation and polymer production in laboratory
The two cultures were then grown on liquid growth medium containing sucrose. The liquid growth medium consisted of 50.0 g sucrose, 2.5 g tryptone, 2.5 g K2 HPO4 and 5.9 g yeast extract per liter of distilled deionized water. The media were transferred to several 1 L Erlenmeyer flasks and sterilized in an autoclave (Tabletop Autoclave, Tuttnauer 2340M, Alpha Scientific, Vancouver, British Columbia, Canada) at a temperature of 121°C and a pressure of 103 KPa for 20 min. Each microbe was transferred aseptically from the nutrient broth to ten 750 mL Erlenmeyer flasks, each having 500 ml of sterilized liquid media. Each flask was inoculated with 10% (v/v) of the homogeneous mixture of the nutrient broth culture. The cultures were grown in a controlled environment laboratory shaker ((MaxQTM 4000 Benchtop Orbital Shaker, Thermo Fisher Scientific, Montreal, Quebec, Canada) at room temperature (21°C) for 5 days. Samples were drawn from the flasks for biomass, sucrose and polysaccharide determination. Sampling was done every 4 h during the first 24 h every 6 h during the period of 24-72 h and then every 12 h until the end of the 5 days. The cell biomass was determined according to the procedure described by Ghaly and Mahmoud [218]. The polysaccharide concentration analysis was determined according to the procedure described by Ramsay [219]. The sucrose concentration was determined according to the procedure described by Borji et al. [220].
Polymer production in soil (Bio-cementing and bio-sealing)
The setup for testing sandy soil bio-cementation and bio-sealing is shown in Figure 7. It consisted of 5 infiltration soil columns, each was constructed a PVC cylinder of 7.5 cm diameter and 40 cm height, a plastic filtration funnel of 7.5 cm diameter and a 1 L flask. The funnel was placed on the top of the flask and a filter bad was placed inside the funnel. This filter bad has a pore size smaller the that of the smallest sand particles (does not allow the soil particles to pass through). The cylinder was connected to the funnel and sealed together. One kg of the sterilized soil was placed in the cylinder and packed to achieve field density. This was done to simulate the soil root zone.
The application of microbial culture and water was carried out as shown in Table 6. 400 ml of microbial culture of Azotobacter chroococcum were added on day 1 to each column. On Day 3, 400 ml of the diluted microbial culture (each soil column received different concentration of the microbial) were added to the columns.
On day 5, the moisture content and pH were measured. The moisture content was measured using a portable soil moisture measurement meter (TOR 150 Soil Moisture Meter, Edaphic Scientific, Moorabbin, Victoria, Australia). The pH was measured using a portable pH meter (Hanna H199121 Digital pH Meter, ITM Instruments Inc, Sainte Anne de Bellevue, Quebec, Canada).Then, 400 ml of water were added to each column and the leachate collected in the flasks were measured after 12, 24 and 48 h from addition of water. Finally, the water holding capacity was determined.
After completing the experiment with Azotobacterchroococcum, the component of each column were dismantled and washed thoroughly with water and disinfected with alcohol. They were the sterilized in an autoclave (Tabletop Autoclave, Tuttnauer 2340M, Alpha Scientific, Vancouver, British Columbia, Canada) at a temperature of 121°C and a pressure of 103 KPa for 20 min. The 5 columns were reassembled again. The same experimental procedure was followed with Lactobacillus fermentum.
RESULTS AND DISCUSION
Soil characteristics
The result showed that the sandy soil used in this study had a particle density of 2.58 g/cm3, a bulk density of 1.6 g/cm3and a porosity of 37.98 %.
The fraction of the soil collected from each sieve as a percentage of the original soil weight is shown in Table 7. The particle size varied from 0.150 to 2.000 mm. Most of the soil particles had a diameter in the range of 0.425-0.850 mm (Figure 8). All sand particles have a diameter within the range 0.05 mm and 2.00, all silt particles have a diameter within the range 0.002 mm and 0.05 mm while all clay particles are less than 0.002 mm in diameter as shown in Figure 9[221].
The results indicated that the soil used in this study is typical sandy soil with a rough texture and free of silt and clay. This soil has a loose texture resulting in wind erosion, low organic matter, low nutrient content, high infiltration rate, low water holding capacity, high temperature resulting in faster plant growth, high aeration rate resulting in faster decomposition of organic matter [222].For a sustainable agriculture, it is important to consider applying biotechnological techniques for building an adequate soil structure in these types of soils as well as water conservation by adopting new irrigation technology.
Bacterial growth and levan production in bioreactor
The bacteria Azotobacter chroococcum and Lactobacillus fermentum were first grown on a sucrose in shake flasks to produce levan. The plate count test performed on the media obtained from the shake flasks containing nutrient broth revealed that there was a count of approximately 7.29 x 108 microbial cells/mL for Azotobacter chroococcum and 8.23 x 108 microbial cells/mL for Lactobacillus fermentum. The result of batch culture propagation of Azotobacter chroococcum and Lactobacillus fermentum in the shake flasksare presented in Table 8.The maximum biomass concentration was 3.6 g/L and 3.0 g/L after 22 h and 26 h for Azotobacter chroococcum and Lactobacillus fermentum, respectively. The concentration of sucrose decreased reaching 1.6 g/L and 1.5 g/L after 56 h and 61 for Azotobacter chroococcum and Lactobacillus fermentum, respectively. With the depletion of sucrose, the bacterial cell mass decreased reaching 0.16 g/L and 0.13 g/ L after 68 h and 72 h for Azotobacter chroococcum and Lactobacillus fermentum, respectively. The bacteria produced the enzyme levansucrase which converts the soluble sucrose into the polysaccharide ß-D fructoside (levan) and glucose. The production of levan reached a maximum of 14 g/L and 17 g/L for Azotobacter chroococcum and Lactobacillus fermentum, respectively.
During the fermentation process, the bacteria utilize sucrose for production of levan and for cell maintenance and growth. The following equations describe product formation, respiration and energy production and growth and reproduction.
(a) Respiration and energy production
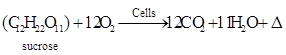 (4)
(4)
(b) Growth and reproduction
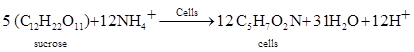 (5)
(5)
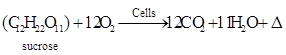 (4)
(4)(b) Growth and reproduction
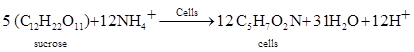 (5)
(5) (c) Product formation
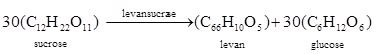 (6)
(6)Equation (4), (5) and (6) can be combined to yield the following equation:
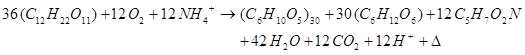 (7)
(7) From equation (7), it appears that the theoretical levan yield is 0.395 g levan/g sucrose and the theoretical cell yield is 0.130 g cells/gsucrose. In this study, the levan yield was 0.248 glevan/g sucrose (62.78% of theoretical yield) and 0.371 g levan/g sucrose (93.92% of theoretical yield) for Azotobacter chroococcum and Lactobacillus fermentum, respectively. The cell yield was 0.074 g cell/g sucrose (47.69 % of theoretical yield) and 0.062 gcell/g sucrose (56.92 % of theoretical yield). The results showed that Lactobacillus fermentum was mor efficient in converting sucrose to levan than Azotobacter chroococcum. However, Azotobacter chroococcum produced more bacterial biomass (g cells/g sucrose) than Lactobacillus fermentum. This may be due to the fact that Azotobacte rchroococcum is a nitrogen fixing microorganism, a process that require organic matter. Thus, some of the sucrose may have been utilized in nitrogen fixation.
The results showed that it is feasible to use growing cultures of Azotobacter chroococcum and Lactobacillus fermentum. From the biological and biochemical characteristics of the Azotobacter chroococcum and Lactobacillus fermentum, it appears that the organisms can produce levan from sucrose under most field and soil conditions and they should be able to compete with most common soil microbial species.
The polysaccharide (levan) produced in this study was non-viscous and water insoluble. The viscosity of the culture broth was the same as that of water. The polymer was a non-transparent suspension and was found to deflect visible light. The polymer can be used as a plugging agent to plug the pores of high permeability soils. Microbial levan contains up to 3 million residues compared to plant levan which contains about 100 residues [51]. The polysaccharide levan (C6H10O5)n consists of fructose monomers linked mainly by β(2→6) linkages [91].
Bio-cementation and bi-sealing
The moisture content and pH measurements taken on day 5 before the application of 400 ml water to each of the soil bio-cementation and bio-clogging columns are presented in Table 9. The results indicated that the moisture content of the soils receiving the bacterial culture of Azotobacter chroococcum (22.3%) was lower than that of the soils receiving the bacterial culture of Lactobacillus fermentum (25.3%). The soils designated as control (received no bacterial treatment) had lower moisture content than the soils treated with both bacterial cultures. The moisture content of the soils receiving the bacterial culture of Azotobacter chroococcum was higher than that of the control by13.78% while the moisture content of the soil receiving the bacterial culture Lactobacillus fermentum was higher than that of the control by 29.59%. However, increasing the concentration of the bacteria cultures that were added on day 3 did not have any significant effect on the moisture content. There was also no change in the soil pH as a result of addition of bacterial cultures or varying the concentration of bacterial culture added on day 3.
The volumes of leachates collected from the soil bio-cementation and bio-clogging experiment after the addition
of 400 ml water for Azotobacter chroococcum and Lactobacillus fermentum are shown in Table 10.
The results showed that increasing the concentration of bacteria from 25 to 100% in the bacterial culture added on day 3 did not have any significant effect on the amount of leachate collect for both bacteria. However, the leachates collected from the soils receiving Azotobacter chroococcum (205 ml) were much larger than those collected from the soils receiving Lactobacillus fermentum (105 ml). Also, the leachates collected from the control (received no bacterial treatment) were much larger (310 ml) than both soils treated with both bacterial cultures. In other words, 90 ml (22.5%), 190 ml (47.5%) and 295 ml (73.75%) of the added water on day 5 were retained by the control, the soil receiving Azotobacter chroococcum and the soil receiving Lactobacillus fermentum, respectively. This amount to a water conservation of 100 ml (25%) and 205 ml (51.25%) for the soil receiving Azotobacter chroococcum and the soil receiving Lactobacillus fermentum, respectively.
The results obtained from the study showed that it is feasible to use growing cultures of Azotobacter chroococcum and Lactobacillus fermentum to produce a water insoluble levan. The polymer can be used as a plugging agent to plug the pores of the high permeability sandy soils. Upon production of levan, pore spaces would be reduced and, hence, the hydraulic conductivity would be substantially reduced. In addition to producing levan, these bacteria also produce gelatinous agents and elemental compounds that cause soil bio-cementation as shown in Figure 12.
The bacteria could be grown in the laboratory either in the non-polysaccharide producing mode or in the polysaccharide producing mode. The first would permit distribution of the bacteria to the lower soil layers but would delay the production of the polysaccharide due to the extension of the lag period required to produce the enzyme (levansucrase).
Improving soil properties using biological techniques such as gleization, bio-grouting or bio-cementation and bio-plugging or bio-sealing has been reported by many authors. Kumariad and Xiang [33] stated that bio-grout is an excellent technique for reducing the permeability of porous soils and improving their mechanical properties. Mujab et al. [34] reported that bio-cementation binds soil particles together leading to increased soil strength and stiffness against wind erosion. Ivanove and Chu [36] evaluated the application of bio-cementation and bi-clogging techniques for reducing the porosity and hydraulic conductivity of soils and found facultative and microaerophilic bacteria to be the most suitable organisms for these techniques. McConkey et al. [40] applied an enhanced gleization technique into irrigation canal and reduced water seepage by 30%. Ghaly [42] developed an enhanced bio-sealing mechanism for earthen manure storage using levan producing microorganism and reported that the infiltration rate was affected by the soil type and was correlated to the percentage of sand in the soil. Knapenetal [47] studied the effect of microbiotic crust on soil erodibility by wind and reported a 37% reduction in soil detachment. Ghaly et al. [49] studied the plugging effect of levan produced by C in earthen manure storage and found the bacteria converted sucrose into levan under field condition and the exopolysaccharide plugged the pores of a highly porous soil. Stewart and Folger [50] used polymer producing bacteria to modify soil profiles for enhanced oil recovery and reported that the bacteria utilized sucrose to produce exopolymer which created plugged regions of the porous media leading to enhanced oil recovery. Ramsay et al. [54] used Bacillus licheniformis to produce water insoluble levan that was used as a selective plugging agent in microbial enhanced oil recovery under a temperature of 55oC, a pH between 6 and 9, a pressure less than sooata and a salt concentration of 4%.
CONCLUSION
The result showed that the sandy soil used in this study had a particle density of 2.58 g/cm3, a bulk density of 1.6 g/cm3and a porosity of 37.98 %. The particle size varied from 0.150 to 2.000 mm with most of the soil particles having a diameter in the range of 0.425-0.850 mm indicating that the soil was free of silt and clay. This soil has a loose texture, low organic matter, low nutrient content, high infiltration rate, low water holding capacity.
Azotobacter chroococcum are capable of producing levan from sucrose and have ability to fix molecular nitrogen and produce growth hormones, and therefore increase the soil fertility and stimulate plant growth, Lactobacillus fermentum bacteria are a levan producing bacteria. The viscosity of the culture broth was the same as that of water. The polymer can be used as a plugging agent to plug the pores of high permeability soils. The levan yield was 0.248 glevan/g sucrose (62.78% of theoretical yield) and 0.371 glevan/g sucrose (93.92% of theoretical yield) for Azotobacter chroococcum and Lactobacillus fermentum, respectively. The cell yield was 0.074 g cell/g sucrose (47.69 % of theoretical yield) and 0.062 g cell/g sucrose (56.92 % of theoretical yield).
The results showed that increasing the concentration of bacteria had not significant effect on the amount of leachate collect for both bacteria. However, the leachates collected from the soils receiving Azotobacter chroococcum were much larger than those collected from the soils receiving Lactobacillus fermentum. Also, the leachates collected from the control (received no bacterial treatment) were much larger than soils treated with both bacterial cultures. These microorganisms can be used together for production of levan from sucrose, fixing nitrogen in soil and producing growth hormones, thereby improving the water holding capacity of the soil and enhancing its nutrient content and stimulating plant growth.
ACKNOWLEDGEMENTS
The project was carried out in the Bioengineering Laboratory of the Department of Agricultural Engineering, Faculty of Agriculture, Cairo. The authors appreciate the assistance provided by the Laboratory Manager Ms. D. M. El Nakib and the Workshop Supervisor Mr. A. M. Abdel Haleem.
COMPETING INTRESTS
The authors have declared that no competing interests exist.
AUTHORS’ CONTRIBUTIONS
This work was carried out in collaboration among all authors. All authors contributed equally in various roles including formulation research goals, development of methodology, performing the experiments and analysing data and writing the initial draft. The corresponding author coordinated the research activity as agreed by all authors. All authors read and approved the final manuscript.
-
- Shafy AH, El Shaharty A, Regelsberger M (2010) Rainwater in Egypt: Quality, distribution and harvesting. Mediterr Mar Sci 11: 245-257.
- Maksoud A (2018) Estimation of temperature and rainfall in Egypt. Asian J Adv Res Rep 1: 1-22.
- Ali WH (2013) Suitability of Egypt deserts for sustainable urban development. Dev Countries Stud 3: 164-173.
- Karasapan O, Shah S (2018) Egypt’s population: Boom then bust. Brookings Institution, Washington, DC, USA.
- CAPMPS (2019) Current and predicted Egyptian population. Central Agency for Public Mobilization and Statistics, Cairo, Egypt.
- MALR (2019) Food export and import. Ministry of Agriculture and land Reclamation, Cairo, Egypt.
- Habib IM, Morsy AA (2003) Land Reclamation and Improvement. Cairo University Open Education Centre. Giza, Egypt (Arabic with English translation).
- DRC (2018) Land reclamation program in Egypt. Desert Research Centre, Ministry of Agriculture and Land Reclamation, Cairo, Egypt.
- Alary V, Aboul-Naga A, Osman MA, Daoud I, Abdelrahman S, et al. (2012) Desert land reclamation program and family desert land dynamics in western desert of the Nile Delta (Egypt), 1960-2010. World Development, 104: 140-153.
- NRCS (1999) Soil taxonomy: A basic system of soil classification for making and interpreting soil surveys. Agriculture Handbook Number 436 (2nd Edition). Natural Resource Conservation Services, United States Department of Agriculture, Washington DC, USA, P869.
- Pankova EI, Gerasimova MI (2012) Desert soils: Properties, pedogenic process and classification. Arid Ecosystems 2: 69-77.
- Suleiman FH, Sadeq SA (1992) Inventory and classification of desert lands. Cairo University Press, Giza, Egypt (Arabic with English translation).
- El Shawarby MU (1961) Soil Chemistry. Anglo-Egyptian Bookshop, Cairo, Egypt (Arabic with English translation).
- Alaily F (1986) Cracks in sandy soils of the extreme arid part of Sahara. Soil Science Hamburg 3: 1023-1024.
- Petit M, Montaigne E, El Hadad-Gauthier F, Alvarez-Coque GMG, Mattas K, et al. (2015) Sustainable agricultural development: Challenges and approaches in Southern and Eastern Mediterranean countries. Cooperative Management, Springer International Publishing, New York, USA.
- Shehata M (2016) Ground water in Egypt: Reality and future prospects. Paper presented at the Physics of the Earth and Treasures Conference, Cairo, Egypt (Arabic with English translation).
- Indraratna B, Chu J (2005) Ground improvement: Case histories. Elsevier Oxford, UK.
- Monnie F (2016) Effect of biochar on soil physical properties, water use efficiency and growth of maize in a sandy loam soil. MPHIL Thesis, University of Ghana Digital collections, Department of Agricultural Engineering, College of Basic and Applied Science, School of Engineering, University of Ghana, Legon Boundary, Accra, Ghana.
- Ding Y, Liu Y, Liu S, Li Z, Tan X, et al. (2016) Biochar to improve soil fertility: Review. Agron Sustain Dev 36: 36-48.
- Jeffery S, Verheijen FGA, Van Der Velde M, Bastos AC (2011) A quantitative review of the effects of biochar application to soils on crop productivity using meta-analysis. Agric Ecosys Environment 144: 175-187.
- Major J, Rondon M, Molina D, Riha SJ, Lehmann J (2010) Maize yield and nutrition during 4 years after biochar application to a Colombian savanna oxisol. Plant Soil 333: 117-128.
- Glaser B, Lehmann J, Zech W (2002) Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal: A review. Biol Fertil Soils 35: 1719-1730.
- Abu Dreeh OEA (2017) Effect of organic matter addition on the growth of Abu Sabeen (Sorghum Bicolor) in sandy soil. Technical Report, University of Sudan for Science and Technology, Khartoum, Sudan (Arabic with English translation).
- Gomez-Sagasti MT, Hernandez A, Artetxe U, Garbisu C, Becerril JM (2018) How valuable are organic amendments as tools for the phyto-management of degraded soils? The Knowns, Known Unknowns, and Unknowns. Front Sustainable Food Sys 2: 1-16.
- Bhogal A, Nicklson FA, Rollet A, Taylor M, Litterick A, et al. (2018) Improvements in the quality of agricultural soils following organic material additions depend on both the quantity and quality of the materials. Appl Front Sustain Food Sys 2: 1-12.
- Lal R (2009) Challenges and opportunities in soil organic matter research. Eur J Soil Sci 60: 158-169.
- Smith P, Lutfalla S, Riley WJ, Torn MS, Schmidt MWI (2017) The changing faces of soil organic matter research. Eur J Soil Sci 69: 23-30.
- Spagnoli G (2018) A review of soil improvement with non-conventional grouts. Int J Geotechnical Eng.
- Kazemian S, Huat BBK, Prasad A, Barghochi M (2010) A review of stabilization of soft soils by injection of chemical grouting. Aus J Basic Appl Sci 4: 58625868.
- Kazemian S, Huat BBK (2009) Assessment and comparison of grouting and injection methods in geotechnical engineering. Eur J Sci Res 27: 234-247.
- Karol RH (2003) Chemical Grouting and Soil Stabilization (3rd Edition). Marcel Dekker, New York, USA.
- Krizek RJ (1985) Chemical Grouting in Soils Permeated by Water. J Geotech Eng 111: 898-911.
- Kumari D, Xiang W (2018) Review on biologically based grout material to prevent soil liquefaction for ground improvement. Int J Geotech Eng 13: 48-53.
- Mujah M, Shahin MA, Cheng L (2017) State-of-the-Art Review of Bio-cementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol J 34: 524-537.
- Wang Z, Zhang N, Cai G, Jin Y, Ding N (2017) Review of ground improvement using microbial induced carbonate precipitation (MICP). Mar Georesour Geotec 35: 1135-1146.
- Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bio-clogging and bio-cementation of soil in situ. Rev Environ Sci Biotechnol 7: 139-153.
- Whiffin VS, van Paasse LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24: 417-423.
- Bockheim JG, Hartemink AE (2017) Soil-forming process. The soils of Wisconsin, Springer International Publishing, New York, USA.
- Bockheim JG, Gennadiyev AN (2000) The role of soil-forming process in the definition of taxa in soil taxonomy and the world soil reference base. Geoderma 95: 53-72.
- McConkey BG, Reimer CD, Nicholaichuk W, Jame YW (1990) Sealing of earthen hydraulic structures with enhanced gleization and sodium carbonate: I. Laboratory study on the effect of freeze-thaw cycle and drying interval. Can Agric Eng 32: 163-170.
- McConkey BG, Reimer CD, Nicholaichuk W, Jame YW (1990) Sealing of earthen hydraulic structures with enhanced gleization and sodium carbonate: II. Application for lining an irrigation canal. Can Agric Eng 32: 171-176.
- Ghaly AE (1989) Enhanced biological sealing for earthen manure storages. Proceedings of the 11th International Congress on Agricultural Engineering, A.A. Balkema Publisher, Amsterdam, pp: 399-408.
- Armanise E, Simmons RW, Ahn S, Garbout A, Doerr SH et al. (2018) Soil sealing development under simulated rainfall: Structural, physical and hydrological dynamics. J Hydrol 556: 211-219.
- Belnap J, Gardner JS (1993) Soil microstructure in soils of the Colorado Plateau - the role of the cyanobacterium Microcoleus vaginatus. West N Am Nat 53: 40-47.
- Bowker MA, Maestre FT, Escolar C (2010) Biological crusts as a model system for examining the biodiversity-ecosystem function relationship in soils. Soil Biol Biochem 42: 405-417.
- Jeffery S, Harris JA, Rickson RJ, Ritz K (2009) The spectral quality of light influences the temporal development of the microbial phenotype at the arable soil surface. Soil Biol Biochem 41: 553-560.
- Knapen A, Poesen J, Galindo-Morales, De Baets S, Pals A (2007) Effects of microbiotic crusts under cropland in temperate environments on soil erodibility during concentrated flow. Earth Surf Proc Land 32: 1884-1901.
- Assouline S, Mualem Y (2000) Modeling the dynamics of seal formation: Analysis of the effect of soil and rainfall properties. Water Resourc Res 36: 2341-2349.
- Ghaly AE, Arab F, Mahmoud NM, Higgins J (2007) Production of Levan by Bacillus licheniformis for use as a soil sealant in earthen manure storage. Am J Biochem Biotechnol 2: 47-54.
- Stewart TL, Fogler HS (2001) Biomass plug development and propagation in porous media. Biotechnol Bioeng 72: 353-363.
- Han YW, Clarke MA (1990) Production and characterization of microbial levan. J Agric Food Chem 38: 393-396.
- Snikanth R, Reddy CHSS, Siddartha G, Ramaiah MJ, Uppuluri KB (2014) Review on production, characteristics and application of microbial levan. Carbohydrate Polym 120: 102-114.
- Park JK, Khan T (2009) Other microbial polysaccharides: pullulan, scleroglucan, elsinan, levan, alternant, dextran. Handbook of Hydrocolloids, Woodhead Publishing, Elsevier Massachusetts, USA.
- Ramsay JA, Cooper DG, Newfeld KJ (1989) Effect of oil reservoir conditions and the production of water- insoluble levan by Bacillus licheniformis. Geomicrobiol J 7: 155-156.
- Chen X, Gao H, Ploehn HJ (2013) Montmorillonite levan nanocomposites with improved thermal and mechanical properties. Carbohydrate Polym 101: 565-573.
- Srikanth R, Siddartha G, Reddy CHS, Harish BS, Ramalah MJ et al. (2015) Antioxidant and anti-inflammatory levan produced from Acetobacter xylinum MCIM2526 and its statistical optimization. Carbohydrate Polymers 123: 8-16.
- Silbir S, Dagbagli S, Yegin S, Baysal T, Goksungur Y (2014) Levan production by Zymomonasmobilis in batch and continuous fermentation systems. Carbohydrate Polym 99: 454-461.
- Jathore NR, Bule MV, Tilay AV, Annapure US (2012) Microbial levan from Pseudomonas fluorescens: Characterization and medium optimization for enhanced production. Food Sci Biotechnol 21: 1045-1053.
- Sarilmiser HK, Oner ET (2014) Investigation of anti-cancer activity of linear and aldehyde-activated levan from Halomonas smyrnensis AAD6T. Biochem Eng J 92: 28-34.
- Shih IL, Yu YT, Shieh CJ, Hsieh CY (2005) Selective production and characterization of levan by Bacillus subtilis (Natto) Takahashi. J Agric Food Chem 53: 8211-8215.
- Zhurina D (2009) Identification and potential characterization of transcriptional regulators involved in temperature-dependent expression of levansucrase in Pseudomonas syringae. PhD Thesis, Jacob University Bermen, Vegesack, Bermen, Germany.
- Youssef GA, Youssef YAS, Talha S, El-Aassar SA (2014) Increased fructosyltransferase (levansucrase) production by optimizing culture condition from Pediococcus acidilactici strain in shaking batch cultures. Life Sci J 11: 33-47.
- Combie J (2006) Properties of levan and potential medical uses. Chapter: 13, Polysaccharides for Drug Delivery and Pharmaceutical Applications, pp: 263-269.
- Dahech I, Belghith KS, Hamden K, Feki A, Belghith H et al. (2011) Antidiabetic activity of levan polysaccharide alloxan-induced diabetic rats. Int J Biol Macromol 49: 742-746.
- Gupta SK, Das P, Singh SK, Akhtar MS, Meena DK, et al. (2011) Microbial levan, an ideal prebiotic and immuno-nutrient in aquaculture. World Aquaculture 61-66.
- Gupta SK, Pal AK, Sahu NP, Saharan N, Mandal SC, et al. (2014) Dietary microbial levan ameliorates stress and augments immunity in Ciprinus cario fry (Linnaeus, 1758) exposed to sublethal toxicity fipronil. Aquac Res 45: 893-906.
- Sezer AD, Kazak H, Öner ET, Akbuğa J (2011) Levan-based nanocarrier system for peptide and protein drug delivery: Optimization and influence of experimental parameters on the nanoparticle characteristics. Carbohydr Polym 84: 358-363.
- Sarilmiser K, Ates O, Ozdemir G, Arga KY, Őner ET (2015) Effective stimulating factors for microbial levan production by Halomonas smyrnensis AAD6T. J Biosci Bioeng 119: 455-463.
- Freitas F, Alves VD, Reis MAM (2011) Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol 29: 388-398.
- Manandhar S, D’Souza N, Vidhate S (2009) Water soluble levan polysaccharide biopolymer electrospum fibers. Carbohydr Polym 78: 794-779.
- Narula N (2000) Azotobacter in Sustainable Agriculture. Vedams Books Ltd, New Delhi, India.
- Loewenberg JR, Reese ET (1957) Observations on microbial fructosans and fructosanases. Can J Microbiol 3: 643-650.
- Moonmangmee S, Kawabata K, Tanaka S, Toyama H, O. Adachi O, et al. (2002) A novel polysaccharide involved in the pellicle formation of Acetobacteraceti. J Biosci Bioeng 2: 192-200.
- Tomulescu C, Stoica R, Secenco C, Casarica A, Moscovici M (2016) Levan: A mini review. Scientific Bulletin Series F: Biotechnology, Vol XX: 309-320.
- Hernandez L, Arrieta J, Menedez C, Vasquez R, Coego R, et al. (1995) Isolation end enzymatic properties of levansucrase secreted by Acetobacter diazotrophicus SRT4, a bacterium associated with sugar cane. Biochem J 309: 113-118.
- Arrieta J, Hernandez L, Geogo A, Suarez V, Balmori E, et al. (1996) Molecular characterization of the levansucrase gene from the endophytic sugarcane bacterium Acetobacter diazotrophicus SRT4. Microbiol 142: 1077-1082.
- Támbara Y, Hormaza JV, Pérez C, León A, Arrieta J (1999) Structural analysis and optimized production of fructo-oligosaccharides by levansucrase from Acetobacter diazotrophicus SRT4. Biotechnol Lett 21: 117-121.
- Batista F, Hemandez RL, Femandez JR, Arrieta J, Menendez C et al. (1999) Substitution of Asp-309 by Asn in the Arg-Asp-Pro (RDP) motif of Acetobacter diazotrophicus levansucrase affects sucrose hydrolysis, but not enzyme specificity. Biochem J 337: 503-506.
- Perumpuli PABN, Watanabe T, Toyama H (2014) Pellicle of thermotolerant Acetobacterpasteurianus strains: characterization of the polysaccharides and of the induction patterns. J Biosci Bioeng 2: 134-138.
- Minakami H, Entani E, Tayama K, Fujiyama S, Masai H (1983) Isolation and characterization of a new polysaccharide-producing Acetobacter sp.. Agric Biol Chem 84: 2405-2414.
- Tayama K, Minakami H, Entani E, Fujiyama S, Masai H (2014) Structure of an acidic polysaccharide from Acetobacter sp. NBI 1022. Biol Chemi 48: 9595-966.
- Jansson PE, Lindberg J, Wimalasiri KS, Dankert MA (1993) Structural studies of acetan, an exopolysaccharide elaborated by Acetobacter xylinum. Carbohydr Res 245: 303-310.
- Wong HC, Fear AL, Calhoon RD, Eichinger GH, Mayer R, et al. (1990) Genetic organization of the cellulose synthase operon in Acetobacter xylinum. Proc Nat Acad Sci USA 87: 8130-8134.
- Gonzalez-Garcinuno A, Tabemero A, Sanchez-Alvarez JM, Galan MA, del Valle EMM (2017) Effect of bacteria type and sucrose concentration on levan yield and its molecular weight. Microb Cell Fact 191: 1-11.
- Bansal A, Singh K, Karnwal A (2019) Effective abiotic factors on production of levan by microorganisms: A review. IJBS 4: 1-6.
- Tabernero A, Gonzalez-Garcinuno A, Sanchez-Alvearez JM, Galan MA, del Vall EMM (2017) Development of a nanoparticle system based on a fructose polymer: Stability and drug release studies. Carbohydr Polym 160: 26-33.
- Pabst MJ (1977) Levan and levansucrase of Actinomyces viscosus. Infect Immun 15: 518-526.
- Warner TN, Miller CH (1978) Cell-associated levan of Actinomyces viscosus. Infect Immun 22: 266-274.
- Miller CH, Somers PJB (1978) Degradation of Levan by Actinomyces viscosus. Infect Immun 15: 518-526.
- Igarashi T, Takahashi M, Yamamoto A, Etoh Y (1987) Purification and characterization of levanase from Actinomyces viscosus ATCC 19246. Infect Immun 552: 3001-3005.
- Han YW (1990) Production and characterization of microbial Levan. Agric Food Chem 38: 393-396.
- Yamasato K, Akagawa M, Oisha N, Kuraishi H (1982) Carbon substitute assimilation profile and other taxonomic features of Alcaligenes faecalis, Alcaligenes and Achromobacter xulosoxidans. JGAM 18: 195-213.
- Srinivasan S, Quastel JH (1958) Enzymatic syntheses of oligo-and polysaccharides containing D-Glucosamine. Science 127: 143-144.
- Wilkinson JF, Dudman WF, Aspinall CG (1955) The extracellular polysaccharide of Aerobacter aerogenes A3 (S1) (Klebsiella type 54). Biochem J 59: 446-451.
- Evans TH, Hibbert H (1946) Advances in Carbohydrate Chemistry, Academic Press, New York, New York.
- Takeshita M (1873) Translucent colony form of the gram-negative, levan-producing bacterium, Aerobacter levanicum. J Bacteriol 16: 503-506.
- Feingold DS, Gehatia M (1957) The structure and properties of levan, a polymer of D‐fructose produced by cultures and cell‐free extracts of Aerobacter levanicum. J Polym Sci 23: 783-790.
- Song KB, Bae KS, Lee YB, Lee KY, Rhee SK (2000) Characteristics of levan fructotransferase from Arthrobacter ureafaciens K2032 and difructose anhydride IV formation from levan. Enzyme Microb Technol 72: 212-218.
- Tanaka K, Karigane T, Nishika F, Yoshida N (1983) Action of levan fructotransferase of Arthrobacter ureafaciens on levan oligosaccharides. J Biochem 94: 1569-1578.
- Tanaka K, Shimonishi M, Kitagaki M, Ikanaga M (1990) Action of levan fructotransferase of Arthrobacter ureafaciens on three oligosaccharides containing a bifurcateresidue. Agric Biol Chem 54: 815-817.
- Hestrin S, Goldblum J (1953) Levanpolyase. Nature 172: 1064-1047.
- De La Vega MG, Celudo FJ, Faneque A (1991) Production of exocellular polysaccharide by Azotobacterchroococcum. Appl Biochem Biotechnol 30: 273-284.
- Han YW (1990) Microbial Levan. Adv Appl Microbiol 35: 171-194.
- Abou-Taleb K, Abdel-Monem M, Yassin M, Draz A (2015) Production, purification and characterization of levanpolymer from Bacillus lentus V8 Strain. Br Microbiol Res J 5: 22-32.
- Tian FS, Karboune, Hill A (2014) Synthesis of fructo-oligosaccharides and oligolevans by the combined use of levansucrase and endo-inulinasein one-step bi-enzymatic system. Innov Food Sci Emerg Technol 22: 230-238.
- Xavier JR, Ramana KV (2017) Optimization of levan production by cold-active Bacillus licheniformis ANT 179 and Fructo-oligosaccharide synthesis by its levansucrase. Appl Biochem Biotechnol 181: 986-1006.
- Kekez BD, Gojgic-Cvijovic GD, Jakovljevic DM, Kojic JS, Markovic MD, et al. (2015) High levan production by Bacillus licheniformis NS032 using ammonium chloride as the sole nitrogen source. Appl Biochem Biotechnol 175: 3068-3083.
- Mamay D, Wahyuningrum, Hertadi R (2015) Isolation and characterization of levan from moderate halophilic bacteria Bacillus licheniformis BK AG21. Process Chem 16: 292-298.
- Larpin S, Sauvageot N, Pichereau V, Laplace J, Auffray Y (2002) Biosynthesis of exopolysaccharide by a Bacillus licheniformis strain isolated from ropy cider. Int J Food Microbiol 77: 1-9.
- Van Dyk SJ, Ah Kee NL, Frost CL, Pletschke BI (2012) Extracellular polysaccharides production in Bacillus licheniformis SVD1 and its immunomodulatory effect. Bioresourc 7: 4976-4993.
- Strube CP, Homann A, Gammer M, Jahn D (2011) Polysaccharide synthesis of the levansucrase SacB from Bacillus megaterium is controlled by distinct surface motifs. J Biol Chem 286: 17593-17600.
- Tanaka K, Kawaguchi H, Ohno K, Shohji K (1981) Enzymic formation of difructose anhydride IV from bacterial levan. J Biochem 90: 1545-1548.
- Zhang T, Li R, Qian H, Mu W, Miao M (2014) Biosynthesis of levan by levansucrase from Bacillus methylotrophicus SK 21.002. Carbohydr Polym 101: 975-981.
- Li R, Zhang T, Jiang B (2014) Purification and characterization of an intracellular levansucrase derived from Bacillus methylotrophicus SK 21.002. Biotechnol Appl Biochem 62.
- Jadan J, Narnoliya LK, Agarwal N, Singh SP (2019) Catalytic biosynthesis of levan and short-chain fructo-oligosaccharides from sucrose-containing feedstocks by employing the levansucrase from Leuconostocmesenteroides MTCC10508. IJBM 127: 486-495.
- Han YW, Watson MA (1992) Production of microbial levan from sucrose, sugarcane juice and beet molasses. J Ind Microbiol 9: 257-260
- Han YW (1989) Levan production by Bacillus polymyxa. J Ind Microbiol 4: 447-452.
- Liu J, Luo J, Ye H, Zeng X (2012) Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus polymyxa EJS-3. Food Chem Toxicol 50: 767-772.
- Jensen SL, Diemer MB, Lundmark M, Larsen FH, Blennow A, et al. (2016) Levanase from Bacillus subtilis hydrolyses β-2, 6 fructosyl bonds in bacterial levans and in grassfructans. Int J Biol Macromol 85: 514-521.
- Ing-Lung S, Yun-Ti Y, Chwen-Jen S, Chien-Yan H (2005) Production and Characterization of Levan by Bacillus subtilis (Natto) Takahashi. J Agric Food Chem 53: 8211-8215.
121.Abdel-Fattah AM, Gamal-Eldeen AM, Helmy WA, Esawy MA (2012) Antitumor and antioxidant activities of levan and its derivative from the isolate Bacillus subtilis NRC1aza. Carbohydr Polym 89: 314-322.
122.Benigar E, Dogsa I, Stopar D, Jamnik A, Cigiæ IK (2014) Structure and dynamics of a polysaccharide matrix: Aqueous solutions of bacterial levan. Langmuir 30: 4172-4182.
123.Ahmed F (2005) Production of levansucrase from Bacillus subtilis NRC 33a and enzymic synthesis of levan and fructo-oligosaccharides. Microbiology 51: 402-407.
- Esawy MA, Ahmed EF, Helmy WA, Mansour NM, El-Senousyand WM (2011) Production of levansucrase from novel honey Bacillus subtilis isolates capable of producing antiviral levans. Carbohydr Polym 86: 823-830.
- Vaidya M, Prasad DT (2012) Thermostable levansucrase from Bacillus subtilis BB04, an isolate of banana peel. J Biochem Technol 3: 322-327.
- Gonçalves BCM, Mantovan J, Ribeiro MLL, Borsato D, Celligoi MAPC (2013) Optimization production of thermo active levansucrase from Bacillus subtilis Natto CCT 7712. J Appl Biol Biotechnol 1: 1-8.
- Abdul Razack S, Velayutham V, Thangavelu V (2013) Medium optimization for the production of exopolysaccharide by Bacillus subtilis using synthetic sources and agro wastes. Turkish J Biol 37: 280-288.
- Abdel-Fattah AF, Mahmoud DAR, Esawy MAT (2005) Production of levansucrase from Bacillus subtilis NRC 33a and enzymatic synthesis of levan and fructo-oligosaccharides. Curr Microbiol 51: 402-407.
- Southerland LW (2004) Microbial Exopolysaccharides: Structure diversity and function versatility of polysaccharides. CRC Press, New York, USA.
- Sucawara M, Haramaki R, Monaka S, Ezura H, Okazaki S (2007) Rhzobiotoxine production in Agrobacterium faciens e58 by Bradyrhizobium elkiirtx ACDEFG genes. Annual Reports of Osaka International Center for Biotechnology, Osaka University, Osaka, Japan.
- Sudtachat, N., N. Ho, S. Eda, H. Mitsui and K.Minamisawa. 2007. Analysis of metabolicfeatures of naturally occurring aromatic compounds in Bradyrhizobium japonicum based on array and gene distribution. Annual Reports of Osaka International Center for Biotechnology, Osaka University, Osaka, Japan.
- Dake, M. 2005. Biodegradable polymers: Renewable nature, life cycle and application. Microbial Factories (V. Kalia Ed), New Delhi, India.
- Rosenberg, e., E. F. DeLong, S. Lory, E. Stackebrandt and F. Thompson. 2014. The Prokaryotes: Alphaproteobacteria and Betaproteobacteria. Springer Publishing Company, New York, USA.
- Liu, Q., S. Yu, T. Zhang, B. Jiang and W. Mu. 2017. Efficient biosynthesis of levan from sucrose by a novel levansucrase from Brenneria goodwinii. Carbohydrate Polymers, 157: 1732-1740.
- Xu, W., Q. Liu, Y. Bai, S. Yu, T. Zhang, B. Jiang and W. Mu. 2017. Physicochemical properties of a high molecular weight levan from Brenneria sp. EniD312. International Journal of Biological Macromolecules, 109: 810-818.
- Gao, S., X. Qi, D. J. Hart, H. Gao and Y. An. 2017. Expression and characterization of levansucrase from Clostridium acetobutylicum. Journal of Agriculture and Food Chemistry, 65 (4): 867-871.
- Dahech, I., H. Ben Ayed, K. S. Belghith, H. Belghith and H. Mejdoub. 2013. Microbial production of levanase for specific hydrolysis of levan. International Journal of Biological Macromolecules, 60: 128-133.
- Dias, F. F. and J. V. Bhat. 1964. Nutritional properties of Corynebacterium laevaniformans. Antone van Leeuwenhoek Journal of Microbiology, 30(1): 176-184.
- Chen, Y. F., Y. N.Yin, X. M. Zhang and J. H. Guo. 2007.Curtobacterium flaccumfaciens pv. beticola, A New Pathovar of Pathogens in Sugar Beet. Plant Disease, 91(6): 667-684.
- Wuerges, J., L. Caputi, M. Cianci, S. Boivin, R. Meijersand S. Benini. 2015. The crystal structure of Erwinia amylovora levansucrase provides a snapshot of the products of sucrose hydrolysis trapped into the active site. Journal of Structural Biology, 191(3):290-8.
- Gross, M., G. Geier, K. Rudolph and K Geidar. 1991. Levan and levansucrase synthesized by the fire blight pathogen Erwinia amylovora. Physiological and Molecular Plant Pathology, 40(6): 371-381.
- Keith, J. A, B. J. Wiley, D. A. Zorfass, S. Arcidiacono, J. M. Mayer and D. Kaplan. 1989. The Production, purification and properties of the biopolymer levan produced by the bacterium Erwinia Herbicola. Technical Report No. 01760-5000, Science and Advanced Technology Directorate, Research, Development and Engineering Center, United States Army, Natick, Massachusetts, USA.
- Keith, J. A, B. J. Wiley, D. A. Zorfass, S. Arcidiacono, J. M. Mayer and D. Kaplan. 1991. Continuous culture system for production of biopolymer Levan using Erwinia herbicola. Biotechnology and Bioengineering, 38(5):557-560.
- Inthanovong L, Tian F, Khodadadi S, Karboune (2013) Properties of Geobacillus stearothermophilus levansucrase as potential biocatalyst for the synthesis of levan and fructo-oligosaccharides. Biotechnol Prog 29: 1405-1415.
- Li Y, Triccas JA, Ferenci T (1997) A novel levansucrase gen cluster in Bacillus stesrothermopholus ATCC12980. Biochem Biophys Act 1353: 203-206.
- Ua-Arak T, Jakob F, R. F. Vogel RF (2017) Fermentation pH modulates the size distributions and functional properties of Gluconobacte ralbidus TMW 2.1191 Levan. Front Microbiol 8: 1-11.
- De Muynck C, Pereora CSS, Naessens M, Parmentier S, Soetaert W, et al. (2007) The genus Gluconobacter oxydans: Comprehensive overview of biochemistry and biotechnological applications. Crit Rev Biotechnol 27: 141-171.
- Jakob F, Rudi DM, Vogel F (2012) Comparison of novel GH 68 levansucrases of levan-overproducing Gluconobacter species. AAB 1(e2): 6-14.
- Velazquez-Hernandez ML, Baizabal-Aguirre VM, Cruz-Vazquez F, Trejo-Contreras MJ, Fuentes-Ramırez LE, et al. (2010) Gluconacetobacter diazotrophicus levansucrase is involved in tolerance to NaCl, sucrose and desiccation, and in biofilm formation. Arch Microbiol 193: 137-149.
- Park NH, Choi HJ, Oh DK (2005) Lactosucrose production by various microorganisms harboring levansucrase activity. Bacteriol Lett 27: 495-497.
- Serrato RV, Meneses CH, Vidal MS, Santana-Filho AP, Iacomini M, et al. (2013) Structural studies of an exopolysaccharide produced by Gluconacetobacter diazotrophicus Pal5. Carbohydr Polym 98: 1153-1159.
- Banguela A, Arrieta JG, Rodríguez R, Trujillo LE, Menéndez C (2011) High levan accumulation in transgenic tobacco plants expressing the Gluconacetobacter diazotrophicus levansucrase gene. J Biotechnol 154: 93-98.
- Kommann H, Duboc P, Marison I, von Stockar U (2003) Influence of Nutritional Factors on the Nature, Yield, and Composition of Exopolysaccharides Produced by Gluconacetobacter xylinus I-2281. Appl Environ Microbiol 69: 6091-6098.
- Sarilmiser HK, Ates O, Ozdemir G, Argaand KY, Oner ET (2015) Effective stimulating factors for microbial levan production by Halomonas smyrnensis AAD6T. J Biosci Bioeng 119: 455-463.
- Poli A, Kazak H, Gürleyendag B, Tommonaro G, Pieretti G, et al. (2012) High level synthesis of levan by a novel Halomonas species growing on defined media. Carbohydr Polym 78: 651-657.
- Brandt JU, Jakob F, Behr J, Geissler AJ, Vogel RF (2016) Dissection of exopolysaccharide biosynthesis in Kozakiabaliensis. Microb Cell Fact 170: 1-13.
- Dutta A, Das D, Goyal A (2001) Purification and characterization of fructan and fructansucrase from Lactobacillus fermentum AKJ15 isolated from Kodo ko jaanr, a fermented beverage from north-eastern Himalayas. Int J Food Sci Nutr 63: 216-224.
- Badel S, Bernardiand T, Michaud P (2011) New perspectives for Lactobacilli exopolysaccharides. Biotechnol Adv 29: 54-66.
- Heinemann C, Johan ET, Veig VH, Janssen DB, Busscher HJ (2000) Purification and characterization of a surface-binding protein from Lactobacillus fermentum RC-14 that inhibits adhesion of Enterococcus faecalis 1131. Biotechnol Lett 90: 117-180.
- Galle S, Avendt EK (2014) Exopolysaccharides from sourdough lactic acid bacteria. Crit Rev Food Sci Nutr 54: 891-901.
- Anwar MA, Kralj S, Pique AV, Leemhuis H, van der Maarel MJEC L (2010) Inulin and levan synthesis by probiotic Lactobacillus gasseri strains: Characterization of three novel fructansucrase enzymes and their fructanproducts. Microbiol 156: 1264-1274.
- Diez-Municio M, de la Rivas B, Jimeno ML, Munoz R, Moreno FJ, et al. (2013) Enzymatic synthesis and characterization of fructo-oligosaccharides and novel maltosylfructosides by inulosucrase from Lactobacillus gasseri DSM 20604. Appl Environ Microbiol 79: 4120-4131.
- Sims IM, Frese SA, Walter J, Loach D, Wilson M, et al. (2011) Structure and functions of exopolysaccharide produced by gut commensal Lactobacillus reuteri 100-23. The ISME Journal 5: 1115-1124.
- Van Hijum SA, Bonting K, van der Maarel MJEC, Dijkhuizen L (2001) Purification of a novel fructosyltransferase from Lactobacillus reuteri strain 121 and characterization of the levan produced. FEMS Microbiol Lett 205: 323-328.
- Kaditzky SV, Vogel RF (2008) Optimization of exopolysaccharide yields in sourdoughs fermented by Lactobacilli. Eur Food Res Technol 228: 291-299.
- Ni D, Xu W, Bai Y, Zhang W, Zhang T, et al. (2018) Biosynthesis of levan from sucrose using a thermostable levansucrase from Lactobacillus reuteri LTH5448. Int J Biol Macromol 113: 29-37.
- Tieking M, Ehrmann MA, Vogel RF, Gänzle MG (2005). Molecular and functional characterization of a levansucrase from the sourdough isolate Lactobacillus sanfranciscensis TMW 1.392. Appl Microbiol Biotechnol 66: 655-663.
- Han, J., X. Xu, C. Gao, Z. Liu and Z. Wu. 2016. Levan-producing Leuconostoccitreum strain BD1707 and its growth in tomato juice supplemented with sucrose. Appl Environ Microbiol 82: 1383-1390.
- Ortiz-Soto ME, Olivares-Illana V, Lopez-Munguia A (2004) Biochemical properties of inulosucrase from Leuconostoccitreum CW28 used for inulin synthesis. Biocatal Biotransfor 22: 275-281.
- Bounaix MS, Gabriel V, Robert H, Morel S, Simeon MR, et al. (2010) Characterization of glucan producing Leuconostoc strains isolated from sourdough. Int J Food Microbiol 144: 1-9.
- Xu Y, Coda R, Shi Q, Tuomainen P, Katina K, Tenkanen M (2017) Exopolysaccharides production during the fermentation of soybean and fava bean flours by Leuconostoc mesenteroides DSM 20343. J Agric Food Chem 65: 2805-2815.
- Liu X, Luo Y, Mohamed OA, Liu D, Wei G (2014) Global transcriptome analysis of Mesorhizobium alhagi CCNWXJ12-2 under salt stress. BMC Microbiology 14.
- Priest PG, Goodfellow M (2000) Applied Microbia Systematics. Springer Science Publisher, New York, USA.
- Kimbrel J (2012) Genome-enabled discovery and characterization of type III effect-encoding genes of plant symbiotic bacteria. Ph D Thesis, Oregon State University, Corvallis, Oregon, USA.
- Reeve W, Yates RJ, Twari R, Gu W (2013) Complete genome sequence of Mesorhizobium austalicum strain type WSM2073T. Stand Genomic Sci 4: 410-419.
- Das K, Rajawat MVS, Saxena AK, Parasanna R (2017) Development of Mesofhizobium ciceri based biofilms and analyses of their antifungal and plant growth promoting activity in cichpea challenged Fusarium wilt. Ind J Microbiol 57: 48-59.
- Chen WX, Li GS, Qi YL, Wang ET, Yuan HL, Li JL (1991) Rhizobium huakuii sp. nov. isolated from the root nodules of Astragalus sinicus. Int J Syst Bacteriol 41: 275-280.
- Kawaharada YS, Eda K, Miamisawa, Mitsui H (2007) A Mesorhizbium loti mutant with reduced glucan content defective invasion of its host plant Lotus japonicus. Annual Reports of Osaka International Center for Biotechnology, Osaka University, Osaka, Japan.
- Kelly SJ, Muszynski A, Kawaharada Y, Hubber AM, Suuivan JT, Sandal N, Carlson RW, Stouguard J, Ronson CW (2013) Controlled requirement for exopolysaccharides in Mesorhizobium-Lotus symbiosis. Mol Plant Microbe Interact 26: 319-329.
- Ray RC (2005) Microbial Biotechnology in Agriculture and Aquaculture. SP Science publishing, Enfield, New Hampshire, USA.
- Bae IY, Oh IK, Lee S, Yoo SH, Lee GH (2008) Rheological characterization of levan polysaccharides from Microbacterium laevaniformans. Int J Biol Macromol 42: 10-13.
- Krichevsky MI, Howell A, Lim JRS (1969) Levan Formation by Odontomyces viscosus. J Dent Res 48: 938-942.
- Xu X, Gao C, Liu Z (2016) Characterization of the levan produced by Paenibacillus bovis sp. nov BD3526 and its immunological activity. Carbohyd Polym 144: 178-186.
- Hang F, Wang Q, Chen W (2017) Effect of oxygen supply on milk-clotting activity expressed by Paenibacillus spp. strain BD3526. Food Sci Technol 82: 437-445.
- Petrov KK, Petrova P (2017) Sugar transport systems involved in fructooligosaccharides utilization by the probiotic bacterium Pediococcu sacidilactici. Compte Rendus de Academic Bulgare des Sciences 70: 1263-1270.
- Haworth N, Stacey M (1940) The chemistry of immunology saccharides. Ann Rev Biochem 17: 97-114.
- Lyne RR, Peat S, Stacey M (1940) Polysaccharides: The constitution of certain levans formed by bacterial action. J Chem Soc 47: 237-244.
- Fuchs A (1956) Synthesis of levan by pseudomonads. Nature 178: 921.
- Alamäe T, Visnapuu T, Mardo K, Mäe A, Zamfir AD (2012) Levan sucrases from Pseudomonas bacteria: Novel approaches for protein expression, assay of enzymes, fructooligosaccharides and hetero-oligofructans. Carbohyd Chem 38: 176-191.
- Al Qaysi SAS (2016) Levan production using Pseudomonas brassicacearum isolated from rhizosphere soil of cowpea farm in Iraq. Iraqi J Biotechnol 15: 83-89.
- Kasapis S, Morris ER, Gross M, Rudolph K (1994) Solution properties of levan polysaccharide from Pseudomonas syringaepv. phaseolicola, and its possible primary role as a blocker of recognition during pathogenesis. Carbohydr Polym 23: 55-64.
- Laue H, Schenk A, Li H, Lambertsen L, Neu TR, Molin S, Ullrich MS (2006) Contribution of alginate and levan production to biofilm formation by Pseudomonas syringae. Microbiol 152: 2909-2918.
- Yoo SE, Yoon E, Cha, Lee H (2004) Antitumor activity of levan polysaccharides from selected microorganisms. Int J Biol Macromol 34: 37-41.
- Kim H, Yang JY, Lee HG, Cha J (2001) Cloning and sequence analysis of a levansucrase gene from Rahnella aquatilis ATCC15552. J Microbiol Biotechnol 11: 693-699.
- Kim HJ, Park HE, Kim MJ, Lee KG, Yang JY, Cha L (2003) Enzymatic characterization of a recombinant levansucrase from Rahnella aquatilis ATCC 15552. J Microbiol Biotechnol 13: 230-235.
- Karunaratne DN (2012) The complex world of polysaccharides. In Tech Open Publishing Company, London, England.
- Tikhonovich IN, Provorov NA, Romanov VI, Newton WE (1995) Nitrogen fixation fundamental and application. Proceedings of the 10th International congress on Nitrogen Fixation, Kluwer Academic Publisher, Norwell, Massachusetts, USA.
- Lesher R, Gerencser VF (1977) Levan production by a strain of Rothia: Activation of complement resulting in cytotoxicity for human gingival cells. J Dent Res 56: 1097-1105.
- Willner SZ, Imam, Hader I (1977) Case Report in Cardiology. 1-3.
- Hill MJ (2018) Microbial metabolism in digestive tract. CRC Press, New York, USA.
- Franken J, Brandt BA, Tai SI, Bauer FF (2013) Biosynthesis of levan: A bacterial polysaccharide, in yeast Saccharomyces cerevisiae. PLoS One 8: 1-14.
- Elorza MV, Villanueva JR, Sentandre R (1977) The mechanism of catabolite inhibition of invertase by glucose in Saccharomyces cerevisae. Biochem Biophys Acta 475: 103-112.
- Ebisu S, Kato K, Kotani S, Misaki A (1975) Structural differences in fructans elaborated by Streptococcus mutans and Streptococcus salivarius. J Biotechnol 78: 879-887.
- Newbrun E, Baker S (1968) Physico-chemical characteristics of the levan produced by Streptococcus salivarius. Carbohyd Res 6: 165-170.
- Moosavi-Nasab MB, Layegh L, Aminlari MB (2010) Microbial production of levan using date syrup and investigation of its properties. Int J Biol Biomol Agric Food Biotechnol Eng 4: 603-607.
- Vigants A, Hicke HG, Marx SP (2001) A simple and efficient method for the purification of membrane-bound levansucrase from Zymomonas mobilis. Curr Microbiol 42: 415-418.
- Calazans GMT, Lima RC, de Franca FP, Lopes CE (2000) Molecular weight and antitumor activity of Zymomonas mobilis levans. Int J Biol Macromol 27: 245-247.
- Bekers MD, Upite E, Kaminska J, Laukevics M, Grube A et al. (2005) Stability of levan produced by Zymomonas mobilis. Process Biochem 40: 1535-1539.
- Melo IR, Pimentel MF, Lopes CE, Calazans GMT (2007) Application of fractional factorial design to levan production by Zymomonas mobilis. Braz J Microbiol 38: 45-51.
- Ananthalakshmy VK, Gunasekaran P (1999) Optimization of levan production by Zymomonas mobilis. Braz Arch Biol Technol 42: 291-298.
- Shaheen S, Aman A, Siddiqui NN (2017) Influence of metal ions, surfactants and organic solvents on the catalytic performance of Levansucrase from Zymomonas mobilis KIBGE-IB14. J Basic Appl Sci 13: 41-46.
- Santos VAQ, Garcia-Cruz VL, Del Bianchi VL (2014) Effect of initial pH in levan production by Zymomonas mobilis immobilized in sodium alginate. Acta Sci Technol 36: 349-354.
- De Oliveira MR, de Silva RSSF, Buzato JB, Celligoi MAPC (2007) Study of levan production by Zymomonas mobilis using regional low-cost carbohydrate sources. Biochem Eng J 37: 177-183.
- Garrity GM, Brenner DJ, Krieg NR, Staley JT (2005) Bergey's Manual of Systematic Bacteriology. Springer Publishing, New York, USA.
- Sneath PHA, Mair NS, Sharpe ME, Holt JG (1986) Bergey’s Manual of Ssystematic Bacteriology. Lippincott Williams and Wilkins, Baltimore, Maryland, USA.
- Breed RS, Murray EGD, Smith NR (1957) Bergey’s Manual of Determinative Bacteriology. The Williams and Wilkins Company: Baltimore, Maryland, USA.
- Ghaly, A. E. and R. M. Ben-Hassan. 1994. Kinetics of batch production of single cell protein from cheese whey. Appl Biochem Biotechnol 50: 79-92.
- Ghaly AE, Mahmoud NS (2006) Optimum condition for measuring dehydrogenase activity of Aspergillus niger using TTC. Am J Biochem Biotechnol 2(4): 186-194.
- Ramsay (1987) Microbial products in enhanced oil recovery. PhD thesis. Department of Chemical Engineering, Faculty of Engineering, McGill University, Montreal, Quebec, Canada.
- Borji AF, Borji, Jourani A (2017) A new method for the determination of sucrose concentration in a pure and impure systems: Spectrophotometric method. Int J Anal Chem 217/8214120: 1-6.
- Schipper L (2013) Soil properties: Relative sizes of sand, silt and clay particles. Science Learning Hub, University of Waikato, Hamilton, New Zealand. Accessed on December 3rd 2019. Available online at: https://www.sciencelearn.org.nz/resources/957-soil-properties
- Braud AC, Hartman, Lesturgez G (2005) Management of tropical soils for sustainable agriculture: A holistic approach for sustainable development of problem soils in the tropics. KhonKaen University Press, KhonKaen, Thailand.











