596
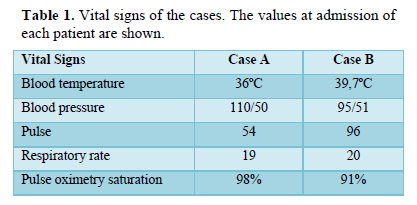
Views & Citations10
Likes & Shares
PATIENTS AND METHODS
Ethical aspects
To prepare case reports, written informed consent was requested from the patients' relatives.
Case A
A 68-year-old female, with a history of hypertension, type 2 diabetes mellitus (NIDDM) (insulin-requiring), chronic kidney disease (CKD) in RRT by means of right jugular HDC (placed in June 2019). She went for assessment on July 15, 2020, for presenting general malaise, fatigue, accompanied by generalized pain (non-specific pain characteristics on questioning) of 15 days of evolution, accompanied by purulent discharge at the HDC insertion site, and epigastralgia. On admission, vital signs without alterations (Table 1), physical examination showed no neurological deterioration, pale face, chest without alterations, painful abdomen in hypogastrium, symmetrical limbs with preserved tone and sensitivity.
Evaluated by a nephrology specialist, to whom she reported thermal rise and discomfort at home prior to admission, which correlated with the last hemodialysis session, establishing presumptive diagnosis of sepsis associated with HDC, indicating empirical scheme with ceftriaxone, after taking blood culture samples. The infectious disease department evaluated her the second day of hospitalization, indicating therapeutic adjustment to meropenem and vancomycin, blood culture sampling from the catheter, removal of the VAD with a change of insertion site and catheter tip culture; additionally, because of risk factors, a cardiological evaluation was agreed upon to consider echocardiography, which would be performed with the results of the blood cultures.
Laboratory results showed leukocytosis, neutrophilia, and elevated acute phase reactants; blood cultures isolated Candida albicans and Staphylococcus aureus (Table 2).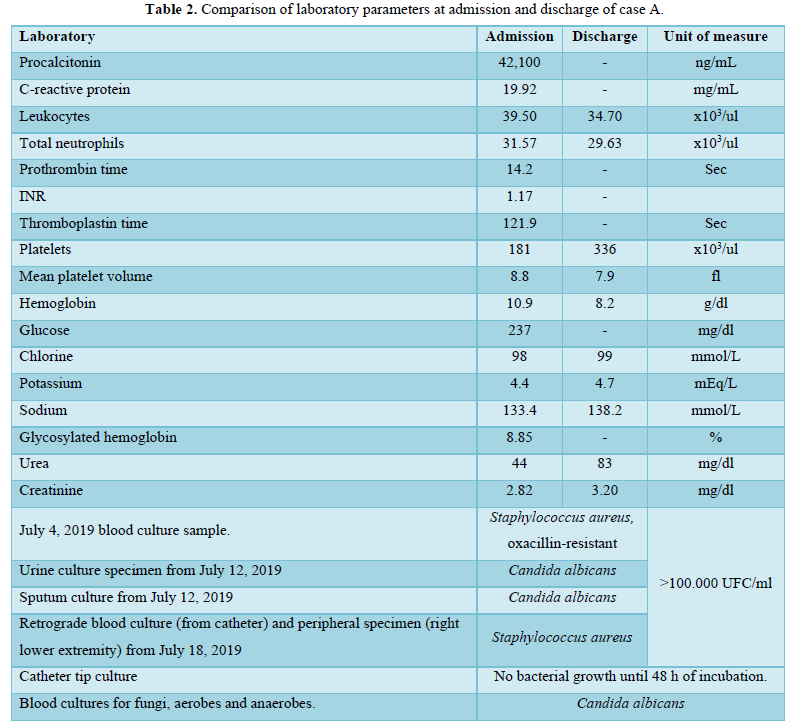
Following the microbiological isolations, an echocardiogram was performed reporting a mass in the right atrium, for which referral to a tertiary care unit was recommended for excision of the mass (for biopsy and culture) and comprehensive management, upon diagnosis of severe mixed sepsis due to coinfection of Candida albicans and Staphylococcus aureus, compounding and vancomycin were indicated, pending biopsy, she was referred to a tertiary level unit, however, upon follow-up of the case, the patient's death was reported without the possibility of performing the biopsy.
Case B
An 82-year-old male, with a history of hypertension, NIDDM and CKD, who started hemodialysis sessions at a private center (December 2021). Attended on January 26, 2022, for presenting 3 days of thermal rise above 40°C, general malaise, asthenia, hyporexia, after a hemodialysis session, classifying his condition as sepsis associated with HDC; levofloxacin and ibuprofen were prescribed; vital signs can be seen in Table 1. Physical examination on admission revealed a foul odor in the vicinity of the HDC insertion site, with erythema, heat and flushing. Upon his deterioration, diagnosis of septic shock associated with HDC was established and the admission to the intensive care unit (ICU) was decided; to establish the diagnosis and therapeutic management of the case, the clinical method was applied. He was admitted to the ICU from January 29 to February 19, 2022, the laboratory results of his evolution can be seen in Table 3, blood cultures were requested for aerobes, anaerobes and fungi, without obtaining samples for lack of institutional reagents and limited economic resources of the family, due to the COVID-19 pandemic.
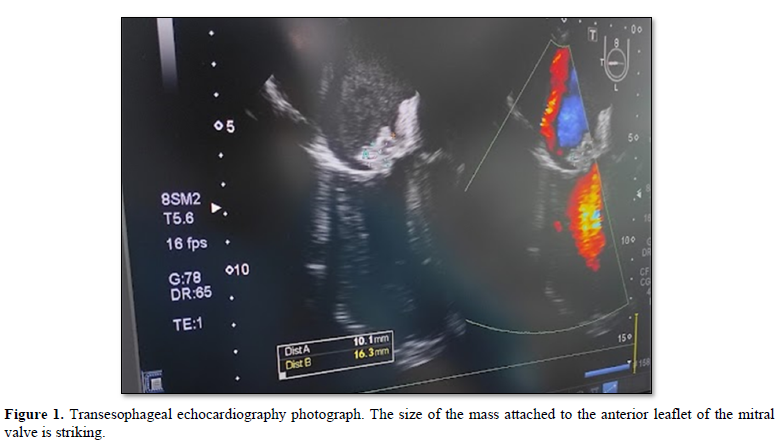
Transthoracic echocardiogram compatible with endocarditis of etiology to be determined reports findings of vegetation affecting the anterior leaflet of the mitral valve of 1.4 x 1.0 cm (Figure 1), generating insufficiency jet running attached to the posterolateral side of the left atrium, additionally, aortic valve calcification with jet of mild tricuspid valvular insufficiency, without record of ventricular dilatation. Concentric hypertrophy, preserved systolic function (55-60%), without regional contractility disorders. Mild bilateral atrial dilatation. No pericardial effusion. Facing a severe sepsis, empirical regimen was started with meropenem and vancomycin adjusted to renal function and hemodialysis session for tentatively 4 weeks; colistin and fluconazole were added after 2 weeks, due to the presumption of fungal infection and probability of infection by strains resistant to carbapenemics; however, the patient’s death was reported without diagnosis of causal microorganism after 4 weeks of antibiotic treatment and 2 weeks of antifungal treatment.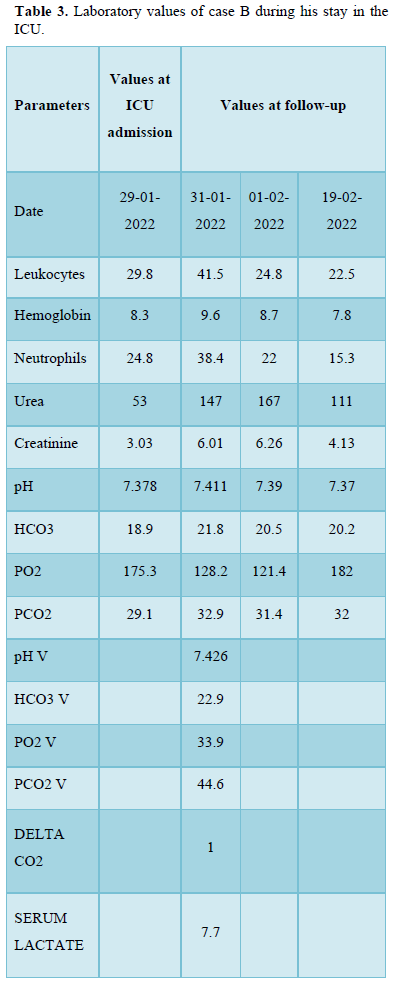
DISCUSSION
VADIs account for 10-20% of all nosocomial infections and complicate the stay of more than 10% of intensive care patients [7]. Given the usefulness of VADs, it is important implementing preventive measures to reduce pathogen exposure in healthcare settings [7-9]. The incidence of VADIs from 1992 to 2004 in the United States ranged from 1.8 to 5.2 events per 1000 days of catheter use (10); while in other parts of the world the incidence can range from 0.48 to 8.75 per 1000 days of device use, and a mortality of 12 to 25% [9]. In both hospital emergency and ICU areas, the microorganism most frequently associated was Staphylococcus aureus [3,8-10].
Presence of infections prior to the use of VADs, mainly due to methicillin-resistant Staphylococcus aureus (MRSA), is considered a highly predisposing factor for reinfection; additionally, the history of NIDDM and duration of the catheter use may condition the development of VADIs [3,10].
Risk factors frequently mentioned as causes of VADIs include device tip colonization, catheter luminal contamination, hematogenous dissemination from a distal infectious focus, and device compromise secondary to device manipulation, drugs or contaminated infusions [8,9]. Microorganisms considered as causal agents include bacterial agents belonging to the usual microbiota of the skin (highlighting S. aureus and coagulase negative staphylococci), as well as transient microbiota (seen in cross-infections in sanitary environments, especially when there is inadequate hand hygiene adherence), Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae and Acinetobacter baumanii; VADIs of fungal etiology has been mentioned, mainly by Candida sp. [8-11]. Among clinical manifestations in patients with VAD are fever, chills and hypotension, allowing presumption of the presence of VADIs, which must be confirmed with the detection of the microorganisms [7-9,12]. Recommendations to reduce VADIs include aseptic measures such as hand hygiene and preparation of the insertion site with iodine-based solutions, 70% alcohol or chlorhexidine gluconate, the removal and change of the device is considered controversial despite reports of therapeutic benefit in several studies and staff training, choice of devices according to the duration of treatment, avoiding femoral access [7,8,13]. Culture of the catheter tip is recommended to confirm colonization of the device (as the cause of the infection), stating that figures higher than 15 CFU in a semiquantitative culture, or higher than 102 on sonication (quantitative study) can be considered as positive [7-9,11,14]. However, microorganism ability to form biofilms and, consequently, the possibility of polymicrobial reports should be evaluated. Therapeutic approach to VADIs involves using empirical and targeted regimens, whose application depends on the clinical conditions of the patient and availability of blood culture isolates. Coverage for gram-positive, gram-negative agents and yeasts should be prioritized, especially when facing signs of septic shock, femoral catheter and prolonged stay in the ICU [7-9,11]. Use of vancomycin is recommended when MRSA infections are suspected, and its substitution by daptomycin when antibiotic minimum inhibitory concentrations (MIC) are higher than 1.5, antifungal therapy and antipseudomonics (carbapenem, piperacillin-tazobactam, etc.) when candidemia or gram-negative agents are suspected in the empirical scheme [7].
- García CA, Caro PV, Quirós CG, Monge BM, Arroyo QA (2020) Catéter venoso central y sus complicaciones. Med Leg Costa Rica 37(1): 74-86.
- Balikci E, Yilmaz B, Tahmasebifar A, Baran ET, Kara E (2020) Surface modification strategies for hemodialysis catheters to prevent C ATHETER‐ R ELATED infections: A review. J Biomed Mater Res B Appl Biomater [Internet]. marzo de 109(3): 314-327.
- Delistefani F, Wallbach M, Müller GA, Koziolek MJ, Grupp C (2019) Risk factors for catheter-related infections in patients receiving permanent dialysis catheter. BMC Nephrol diciembre de 20(1): 199.
- Knezevic V, Djurdjevic MT, Bozic D, Strazmester MG, Mitic I, et al. (2018) Risk factors for catheter-related infections in patients on hemodialysis. Vojnosanit Pregl 75(2): 159-166.
- Schwanke AA, Danski MTR, Pontes L, Kusma SZ, Lind J (2018) Central venous catheter for hemodialysis: incidence of infection and risk factors. Rev Bras Enferm mayo de 71(3): 1115-1121.
- Zuo X, Liu Y, Cai X, Zhan L, Hu K (2022) Association of different Candida species with catheter‐related candidemia, and the potential antifungal treatments against their adhesion properties and biofilm‐forming capabilities. J Clin Lab Anal 35(4): e23738.
- Chaves F, Garnacho MJ, Pozo JL, Bouza E, Capdevila JA, et al. (2022) Diagnosis and treatment of catheter-related bloodstream infection: Clinical guidelines of the Spanish Society of Infectious Diseases and Clinical Microbiology and (SEIMC) and the Spanish Society of Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC). Med Intensiva 42(1): 5-36.
- Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S (2014) Catheter-related bloodstream infections. Int J Crit Illn Inj Sci 4(2): 161.
- Pagani JL, Eggimann P (2008) Management of Catheter-related Infection [Internet]. Medscape. Available online at: https://www.medscape.com/viewarticle/571265
- Sahli F, Feidjel R, Laalaoui R (2017) Hemodialysis catheter-related infection: rates, risk factors and pathogens. J Infect Public Health 10(4): 403-408.
- Bouza E, Burillo A, Muñoz P (2022) Catheter-related infections: diagnosis and intravascular treatment. Clin Microbiol Infect 8(5): 265-274.
- Miller LM, Clark E, Dipchand C, Hiremath S, Kappel J, et al. (2016) Hemodialysis Tunneled Catheter-Related Infections. Can J Kidney Health Dis 3: 205435811666912.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, et al. (2011) Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis 52(9): e162-e193.
- Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, et al. (2009) Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis 49(1): 1-45.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Allergy Research (ISSN:2642-326X)


