2641
Views & Citations1641
Likes & Shares
Allergy is serious healthcare problem that affects
large populations in all countries. Allergic disorders are a multifactorial
disease with a wide spectrum of clinical manifestation and symptoms.
The aim of the present study was to investigate the
immune status and analysis if the allergens can cause in vitro cell activation and membrane blebbing.
The study was used blood of healthy persons and
persons with allergies, allergens: 5% solutions of glucose, sucrose, fructose,
methylene blue dye. Blood cells were placed in a saline solution and subjected
to alternating microelectrophoresis.
When comparing the blood of healthy patients and
patients with allergies, it was found that the patient's healthy cell activity
(CA) above, blebbing and aggregation hardly observed (CA=0.92). When introduced
into a patient's blood glucose allergy (CA=0.6) and sucrose (CA=0.7) was
observed enhanced blebbing and sludge erythrocyte hemolysis them - is an
allergic reaction. Fructose such phenomena have been identified (CA=0.83).The
study was conducted analysis of two leukocytic formulas: before and after
application of riboxin. As a result, changes were slight (1-2%), shifts in the
formula is almost not observed. Then compared the effects of antihistamine on
blood cells. It was found that Suprastin increases the resistance of cells to
the action of the allergen and improves their activity, alters the shape of the
erythrocyte. Some red blood cells bind to monocytes and neutrophils and
blebbing and aggregation not observed.
As a result, the bioelectrical properties of red
blood cells are reduced in case of allergy. In the presence of the allergen,
blood cells become less active, changing the shape and size observed blebbing
lymphocytes and neutrophils, erythrocytes aggregation. Antihistamines increase
cytophysiological characteristics of blood cells of patients with allergies.
Keywords: Immune
status, Allergy, Cell activity (CA), Blebbing
INTRODUCTION
Allergy is serious healthcare problem that
affects large populations in all countries [1]. The prevalence of them has
dramatically increased last decades, with accompanying social costs due to
increase morbidity and lost productivity from missed work or school. Allergic
disorders are a multifactorial disease with a wide spectrum of clinical
presentations and symptoms [2].
The prevalence of asthma in the United States
8.4% has asthma as compared with 4.3% of the population worldwide. The average
annual asthma prevalence is higher in children in comparison to adults (9.5%
vs7.7%) [3,4]. Atopic dermatitis (AD) is a chronic inflammatory skin disease
than affects up to 20% of children and up to 3% of adults worldwide [3].
Allergic rhinitis affects 20 to 30% of adults in both the United States and
Europe [5]. Allergic eye diseases are usually manifested as conjunctivitis with
or without keratitis, in response to an allergen, and affect 10%-20% of people
globally [6].
The etiology of allergic disorders is complex
and multi-faceted [7,8]. Previous studies have indicated that several
environmental factors such as air pollution and smoking, nutritional habits,
chronic stress, genetics are key factors in asthma pathogenesis [2,9].
Moreover, it was demonstrated by previous studies than human microbiota, both
intestinal and upper airway, play an important role in mediating the
pathogenesis of childhood asthma [10].
Blebs are blister like, typically
spherical protrusions that
Based on previous results, the aim of the
present study was to investigate if the allergens can cause in vitro cell activation and membrane
blebbing.
MATERIALS AND METHODS
The study was performed in vitro. For the experiments, 2 ml of heparinized whole blood were
collected from patients with allergy disorders and age matched control
individuals. Blood cells were placed in a 0.9% NaCl solution and exposed to
alternating microelectrophoresis. At the same time, the electrokinetic
properties of cells placed in an alternating constant electric field created by
the “Cyto-Expert” apparatus are carried out as descripted previously [21]. To
assess the electrokinetic properties of the cells, the amplitude of their vibrations
was evaluated. Moreover, percentage of active cells was estimated. Next was
carried out analytical processing of the data and leaving the formulas using
the software [21].
Mediators and
conditions used for stimulation/inhibition studies
To study blebing induction, as well as total
cell activity, blood cells were treated with allergens glucose, sucrose,
fructose (1:20 dilution) for 30 min at 37°C. To investigate the mechanism of
bleb retraction and inhibition of cell activating, human cells were pre-treated
with riboxin and chloropyramine hydrochloridein different concentrations
(primary and diluted in 10%).
RESULTS
Blood cell activation and blebbing formation
Considering
that glucose and sucrose can activate blood cells in patients with allergy, red
blood cells blotting and sludge was observed. On the other hand, similar data
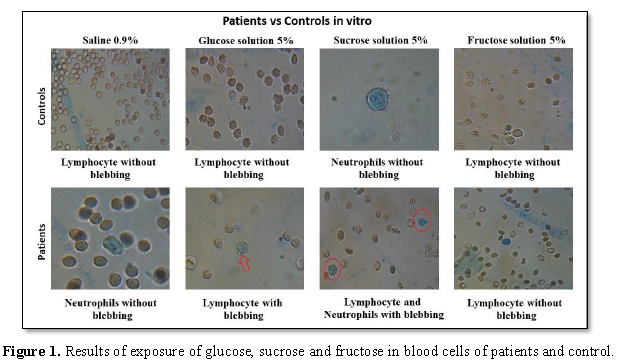
were not observed after cells' exposure to fructose (Table 1).
Moreover, blebbing was observed in patients’ blood cells after exposure to glucose and sucrose, but not detected tin fructose exposure (Figure 1). Blebbing is one of the initial manifestations of cell damage caused by hypoxia, intoxication, the action of viruses, but not leading to cell death [4]. Sludge is a phenomenon characterized by adhesion, aggregation and agglutination of the fomrenic elements of the blood.
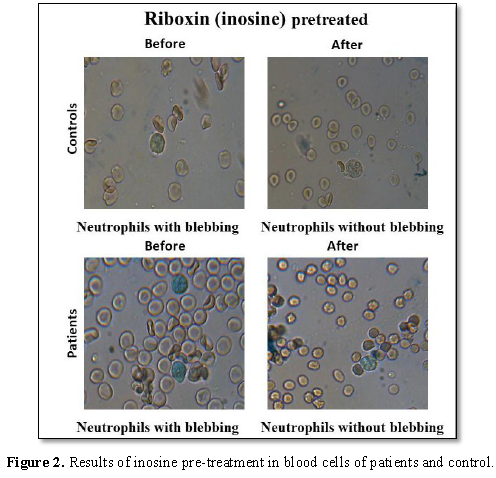
Riboxin restores the in vitro cells activity and inhibit blebbing Riboxin (inosine) - purine nucleoside, the precursor of ATP. Riboxin is involved in metabolic processes, increases stamina, strengthens the immune system and stimulates biochemical processes in muscle tissue, increases protein synthesis. During the research, we analyzed two leukocyte formulas: before and after taking Riboxin. As a result, the changes were insignificant (1-2%); no changes were observed in the formula. After inosine pre-treatment, it was observed that cell activity was increased, blebbing was decreased and there was no sweeping observed (Figure 2).
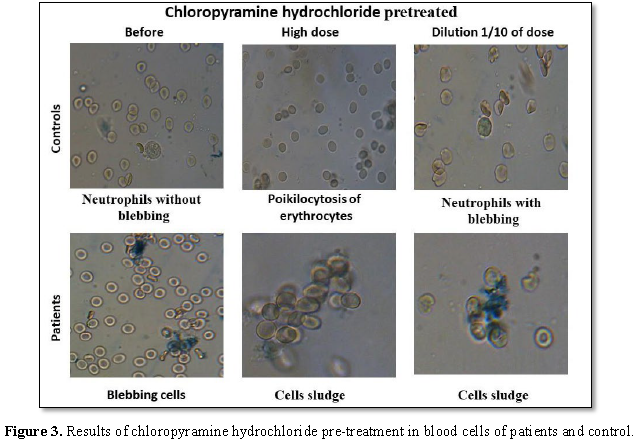
Chloropyramine hydrochloride reduces the in vitro cell activity. We next examined whether chloropyramine hydrochloride, a known antihistamine drug, could reduce the in vitro cell activation by glucose or sucrose. The chloropyramine hydrochloride (Suprastin) is a classic antihistamine drug belonging to the group of ethylenediamine preparations. When conducting research, we compared the effect of different concentrations of Suprastin on blood cells. It was found that a high concentration of antihistamine drug leads to inactivation of cells, loss of membrane potential. There is a tendency to sludge, increases blebbing, lymphocytes die. The normal concentration of suprastin (dilution 10 times) improves the activity of the cells, changes the shape of red blood cells. Some red blood cells bind to monocytes and neutrophils. Thus, an overdose with an antihistamine can lead to a negative effect (Figure 3 and Table 2).
As a result of the
work, we determined that allergy reduces the protective reactions of the body.
In the presence of an allergen, cells reduce activity, change shape and size.
Antihistamines increase the body's resistance, improve blood counts. That is
why in surveys improving immune status is important for every person.
DISCUSSION AND CONCLUSION
We have used the
electrokinetic properties of cells placed in an alternating constant electric
field created by the “Cyto-Expert” apparatus is carried out as descripted
previously [21] to study the immune cells activation and formation of blebs.
There is no doubt, that allergen-driven specific cell activation is critically
required for allergic immune response. In the present experimental model, we
shown that in the presence of an allergy, the protective abilities of the body
are reduced, they are constantly wasted on fighting antigens. Interaction of
allergen and immune response was studied by Borishe [22]. Moreover, Eckl-Dorna
et al. [23] in their experiments have shown that cell activity is higher in a
healthy patient, there was almost no blebbing and sludge.
Blebbing is one of
the initial manifestations of cell damage caused by hypoxia, intoxication, the
action of viruses, but not leading to cell death. Using blebs as detectors of
cells activation in conjunction with riboxin treatment we were able to provide
unequivocal proof of the experience that riboxin can inhibit allergen induced
cell activation. Indeed, riboxin pretreated cells, did not form bleb. Several in vitro and in vivo studies suggest that riboxin has an immunomodulatory and
cell protective action [23,24]. Lazareva and Brovkina [25] in their animal
model study in rats, have shown that riboxin, in combined injection with
Essentiale and Elkar, restores activity of immune cells, hepatocytes and
myocytes, that induced by phenylhydrazine. Additional studies are required to
confirm our preliminary data that riboxin reduces of the allergen-inducted cell
activation that may have clinical relevance. The role of the molecular
mechanisms which induces the blebbing and their biological effects need further
investigations.
Potential
limitations of our study were that we had a small sample size of patients.
Moreover, we do not use in our in vitro
experiments glucocorticoids which play an important role in allergy therapy.
1. Campbell DE, Mehr S (2015) Fifty
years of allergy: 1965-2015. J Pediatr Child Health 51: 91-93.
2. Afanasiev YI, Yurina N, Kotovskij
EF (2012) Histology, embryology, Cytology. 6th Edn, p: 800.
3. Asher MI, Montefort S, Björkstén
B, Lai CKW, Strachan DP, et al. (2006) Worldwide time trends in the prevalence
of symptoms of asthma, allergic rhinoconjunctivitis and eczema in childhood:
ISAAC Phases One and three repeat multi-country cross-sectional surveys. Lancet
368: 733-743.
4. Loftus PA, Wise SK (2016)
Epidemiology of asthma. Curr Opin Otolaryngol Head Neck Surg 24: 245-249.
5. Hoyte FCL, Nelson HS (2018) Recent
advances in allergic rhinitis. F1000Res 7.
6. Patel DS, Arunakirinathan M,
Stuart A, Angunawela R (2017) Allergic eye disease. BMJ 359: j4706.
7. Kazmirchuk VE, Koval’chuk LV,
Maltsev DV (2009) Clinical immunology and Allergology.
8. Byce YV, Butenko GM, Gogenko AI
(2015) Pathophysiology: Textbook.
9. Russell RJ, Brightling C (1979)
Pathogenesis of asthma: Implications for precision medicine. Clin Sci (Lond)
131: 1723-1735.
10. Kozik AJ, Huang YJ (2018) The
microbiome in asthma: Role in pathogenesis, phenotype and response to
treatment. Ann Allergy Asthma Immunol 122: 270-275.
11. Charras G, Paluch E (2008) Blebs
lead the way: How to migrate without lamellipodia? Nat Rev Mol Cell Biol 9:
730-736.
12. Charras GT, Coughlin M, Mitchison
TJ, Mahadevan L (2008) Life and times of a cellular bleb. Biophys J 94: 1836-1853.
13. Fishkind DJ, Cao LG, Wang YL
(1991) Microinjection of the catalytic fragment of myosin light chain kinase
into dividing cells: Effects on mitosis and cytokinesis. J Cell Biol 114:
967-975.
14. Mills JC, Stone NL, Erhardt J,
Pittman RN (1998) Apoptotic membrane blebbing is regulated by myosin light
chain phosphorylation. J Cell Biol 140:627-636.
15. Chikina AS, Svitkina TM,
Alexandrova AY (2018) Time-resolved ultrastructure of the cortical actin
cytoskeleton in dynamic membrane blebs. J Cell Biol 218: 445-454.
16. Taneja N, Burnette DT(2019) Myosin
IIA drives membrane bleb retraction. Mol Biol Cell 30: 1051-1059.
17. Gebala V, Collins R, Geudens I,
Phng L-K, Gerhardt H (2016) Blood flow drives lumen formation by inverse
membrane blebbing during angiogenesis in
vivo. Nat Cell Biol 18:443-450.
18. Nikki Jo-Hao Weng, Cindy Cheung,
Prue Talbot (2018) Dynamic blebbing: A bottleneck to human embryonic stem cell
culture that can be overcome by laminin-integrin signaling. Stem Cell Res 33:
233-246.
19. Coleman ML, Sahai EA, Yeo M, Bosch
M, Dewar A, et al. (2001) Membrane blebbing during apoptosis results from
caspase-mediated activation of ROCK I. Nat Cell Biol 3: 339-345.
20. Barros LF, Kanaseki T, Sabirov R,
Morishima S, et al. (2003) Apoptotic and necrotic blebs in epithelial cells
display similar neck diameters but different kinase dependency. Cell Death
Differ 10:687-697.
21. Soloviev A (2001) Method of
microelectrophoresis of blood cells and epithelial cells and device for its
implementation.
22. Borish L, Joseph BZ (1992)
Inflammation and the allergic response. Med Clin North Am 76: 765-787.
23. Eckl-Dorna J, Villazala-Merino S,
Linhart B, Karaulov AV, Zhernov Y, et al. (2018) Allergen-specific antibodies
regulate secondary allergen-specific immune responses. Front. Immunol 9: 3131.
24. Laskova IL, Uteshev BS (1992) The
immunomodulating action of riboxin during physical loading. Eksp Klin Farmakol
55: 48-50.
25. Lazareva GA, Brovkina IL (2006)
The protective action of essentiale, riboxin and elkar against hemotoxic
anemia. Eksp Klin Farmakol 69: 48-51.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- BioMed Research Journal (ISSN:2578-8892)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Pathology and Toxicology Research