2870
Views & Citations1870
Likes & Shares
Since described as early has 1869 by Knapp,
macular hole has been an entity of great interest for various investigators.
This resulted in continuous revolutions in the underlying pathogenesis and its
management. Recently OCT has emerged as most important imaging modality for
prognostication and planning of surgical intervention. Most popular surgical
intervention to treat macular hole is pars plana Vitrectomy with internal
limiting membrane peeling with gas tamponade. This review article is focused on
clinical features, pathogenesis, roles of newer imaging tools in the management
of macular holes and different surgical approaches.
Keywords: Macula
hole, Gas tamponade, ILM peeling, Brilliant blue G
Abbreviations: MH:
Macular Hole; ERM: Epiretinal Membrane; CME: Cystoid Macular Edema; RD: Retinal
Detachment; CNVM: Choroidal Neovascular Membrane; FTMH: Full Thickness Macular
Hole; SD-OCT: Spectral Domain-Optical Coherence Tomography; FFA: Fundus
Fluorescein Angiography; LMH: Lamellar Macular Holes; HFF: Hole Form Factor;
FAF: Fundus Autofluorescence; LF: Melanolipofuscin; RPE: Retinal Pigment
Epithelium; OPL: Outer Photoreceptor Layer; MPH: Macular Pseudoholes; FP:
Foveal Pseudocysts; VMT: Vitreomacular Traction; ILM: Internal Limiting
Membrane; BBG: Brilliant Blue G; PFCL: Perfluorocarbon Liquid; FDP: Face Down
Posturing; SANFL: Swelling of the Arcuate Retinal Nerve fiber Layer; DONFL:
Dissociated Optic Nerve fiber Layer
INTRODUCTION
Macular hole (MH) represents a partial or
full thickness defect or dehiscence in the central retina at the umbo [1]. The
prevalence rate of MH in India has been found to be 0.17% [2]. With the better
understanding of pathogenesis and improvement in vitreoretinal surgical
technique and instrumentation, excellent visual outcomes can be achieved.
CAUSES
Primary cause of MH in majority of cases is
idiopathic. Trauma is among the most common secondary cause of macular hole.
Apart from trauma, other conditions that can secondarily lead to MH are
epiretinal membrane (ERM), cystoid macular edema (CME), retinal detachment(RD),
proliferative diabetic retinopathy, severe hypertensive retinopathy, choroidal
neovascular membrane (CNVM), juxta foveal telengiectasia, retinoschisis,
lightening, photic retinopathy (electrocution, welding, accidental Nd-YAG
laser) [3-6].
CLINICAL FEATURES
Idiopathic MH usually occurs in the sixth to
seventh decade and women are affected more often than men; reported ratio is
2-3:1 [3]. There is no proven theory for female preponderance but recently
study done with SD-OCT has shown that females has significantly thinner central
foveal thickness [7]. There is 3-29% risk of fellow eye getting affected with
MH [8].
SYMPTOMS
Patients with smaller MH may have no symptoms
and are diagnosed on routine ophthalmoscopic evaluation. Symptomatic patients
usually complain of blurred vision and metamorphopsia. Those with larger holes
will have scotoma or a defect in central vision.
SIGNS
Visual acuity of the affected eye may vary
according to the size, duration, location and associated sub retinal cuff of
fluid. In smaller holes it may vary from 20/25-20/40 while in larger holes it
may 20/80 to 20/400.
Amsler Grid is of great value in which the
patient appreciates the bending/ waviness of lines and scotomas. On fundus
examination, macular hole can be seen as well-defined excavation at the macula
and choroidal reflex can be seen through it. In some cases, few yellowish
deposits can be seen at the base of the hole suggestive of lipofuscin-laden
macrophages. Additionally, surrounding sub retinal fluid can be appreciated, if
present.
The
Watzke-Allen test can be done on slit lamp and with direct ophthalmoscope. Herein,
a thin narrow vertical beam of light is projected onto the macula and the
patient is asked to perceive the light carefully and is asked to draw it on a
paper. In a full thickness macular hole (FTMH), the line drawn is broken.
Narrowing or thinning is suggestive of small MH, partial thickness MH or other
differential diagnosis. A simple test using Maddox rod also reveals broken line
suggestive of FTMH.
The
laser aiming beam test is performed with 50 µm spot size laser-aiming beam.
Test is considered positive when the patient fails to detect the aiming beam
placed within the lesion but is able to detect it when placed onto normal
retina. This test is useful in detection of small MH where Watzke-Allen sign is
negative.
INVESTIGATIONS
Fundus
fluorescein angiography (FFA)
FFA
reveals transmitted fluorescence due to window defect at the area MH. FFA may
also be helpful in prognostication; if done prior to surgery to look for the
perfusion status of macula and anatomy of foveal avascular zone (FAZ).
Optical
coherence tomography (OCT)
Only
28% of lamellar macular holes(LMH) diagnosed on OCT examination were detected
clinically on fundus examination [9] On the basis of changes noted on OCT,
International Vitreomacular Traction Study Group proposed a new classification
system of MH (Table 1) [10]. This
classification is of clinical importance because it determines the management
and prognosis of macular holes. With the advent of ultra-high resolution OCT,
LMH has been described as any of the following (1) an irregular foveal contour;
(2) a break in the inner fovea; (3) separation of the inner from the outer
foveal retinal layers, leading to an intraretinal split; (4) absence of a full
thickness foveal defect with intact photoreceptors posterior to the area of
foveal dehiscence [11].
OCT
BASED PROGNOSIS
Various
parameters as measured by OCT: base diameter, defect depth, central foveal
thickness and perifoveal thickness help to prognosticate the subtypes of LMH.
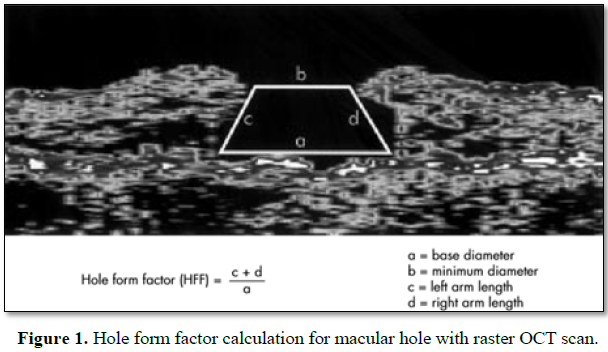
Hole form factor (HFF): It is defined as the ratio of the sum of the lengths of
the two sides of macular hole to the base diameter. HFF between 0.9-1 has been
correlated with better anatomical and functional outcome after surgery whereas
HFF less than 0.5 are found to have poor prognosis (Figure 1) [12].
FUNDUS
AUTO FLUORESCENCE (FAF)
Increased
FAF signal at the base of MH has been attributed to the presence of
melanolipofuscin (LF) or changes in the metabolic activity of the retinal
pigment epithelium (RPE). Decreased FAF signal, suggesting the absent or
degenerating RPE cells with reduced LF granule content [13]. Thus increased
auto fluorescence is a good prognostic factor.
PATHOGENESIS
The pathogenesis is incompletely understood
[14]. A number of theories have been put forward to explain the pathogenesis
[15] (Table 2).
The
major milestone in understanding of pathogenesis of MH was classification by
Gass [1]. Green proposed that chronic low-grade traction due to ocular rotation
stimulates cellular proliferation of Muller cells, astrocytes and RPE
realigning vitreous fibres and redirecting the fractional force in tangential
direction [16].
Studies
done with OCT has showed that the initial stage of MH formation starts as a
triangular elevation of outer photoreceptor layer(OPL) and its detachment from
RPE due to fractional forces. The traction on the fovea occurring prior to
anatomic changes to the fovea has been referred to as Stage 0 and may resolve
without progression in 40-50% of patients [17]. Ezra published an OCT
documented study suggesting that failure of normal age-related separation of
cortical vitreous from posterior pole as a result of an abnormally tenacious
attachment to the fovea leads to MH formation [18].
DIFFERENTIAL
DIAGNOSIS
The
varied presentation of lamellar macular defect may mimic a MH. Lamellar macular
defects were categorized into three different subtypes based on their OCT
appearance: LMH, macular pseudo holes (MPH) and foveal pseudo cysts (FP) [19].
Lamellar
holes have thin fovea resulting from avulsion of inner layer of macula.
A
macular pseudo hole results from the centripetal contraction of an ERM that
subsequently leads to verticalisation of the foveal slopes and a sharply
punched out defect [20].
FP
is described as a precursor to MH or LMH formation due to direct vitreomacular
traction (VMT) [9] ERM can also result in avulsion forces leading to the
formation of pseudo cyst [21].
It
has been found that MPH have smaller diameter and thicker central foveal
tissue, therefore they have better visual acuity than LMH and FP whereas both
LMH and FP are shown to have deeper and wider intraretinal split and also thin
central foveal tissue [19].
MANAGEMENT
FTMH
were once considered untreatable and surgery was indicated once extensive RD
occurred [22,23]. In 1991, Kelly and Wendel first demonstrated a surgical
procedure to close idiopathic MH with good functional outcome [24]. Later
several adjuncts like TGF-β, autologous platelet concentrate, bovine thrombus,
laser barrage, etc. [25,26], currently indications of surgery are as follows:
A. On
the basis of macular hole staging and pre-op visual acuity.
1. FTMH
with stage II and above.
2. Patients
with stage III and IV with visual acuity of 6/18 or below: these patients
readily gain 2 or more line improvement after surgery.
3. Stage
II macular holes with visual acuity ranges from 6/12 to 6/18 (20/40-20/60).
4. Patients
with visual acuity of >6/12 presenting with minimum symptoms like
metamorphopsia and smaller hole size rarely requires surgery. Freeman et al
observed spontaneous regression in 4% of all cases. Therefore, these patients
can be followed up [27].
B. As
per imaging modalities.
OCT
based parameters and FAF are important to prognosticate and to plan the surgery.
SURGICAL
OBJECTIVE OF MACULAR HOLE SURGERY
Surgical objective of MH surgery is
twofold; first to relieve tractional forces; second to activate reparative
healing mechanism [28,29].
Surgical
technique
Pars Plana Vitrectomy with internal
limiting membrane (ILM) peel with fluid gas exchange (FGE) is the accepted
technique. Earlier, 20G system and currently 27G, 25G and 23G systems have been
employed [30]. Brilliant blue G (BBG) dye is used at concentration of 0.25 mg/ml
for staining ILM. This dye does not stain ERM thus after removal of ERM, dye
should be re-injected to look for residual ILM. This method is called as double
staining and ensures complete removal of ILM [31]. Lifting of ILM has been a
challenge as well as traumatic to retina [32]. Newer instruments like silicone
tipped cannula; diamond duster and finesse flex loop have made ILM peeling
convenient and less traumatic.
Recently many techniques have been proposed
for large and refractory MH; fovea sparing ILM, free flap, inverted ILM flap
with and without PFCL, cabbage technique [33-37]. All these techniques aim at
stuffing macular hole with ILM. This is postulated that this ILM will serve as
scaffold for retinal tissue to grow upon. There are various reports that these
flaps get dislocated with fluid current and during fluid air exchange.
Autologous retinal transplant has also been used to plug macular hole also
provides scaffold and plugs macular hole [38].
To
peel or not to peel
Studies favoring ILM peeling state that
peeling removes the template upon which the glial cells proliferate. Also, it
removes the tangential traction. ILM peeling serves to increase MH edge
mobility and reduce MH diameter [29].
Studies which do not advocate ILM peeling
postulated that removal of ILM injures the Müller cell footplates and trigger
reparative gliosis [32].
ILM peeling has shown higher rate (92%) of
primary closure of MH as compared with eyes undergoing MH repair without ILM
peeling (82%). Late reopening of hole was also found to be higher in no ILM
peeling group when compared with ILM peeling group (7% vs. 0.6 %) [39].
To
posture or not to posture
is still a controversial issue. Studies have reported strict face down
posturing (FDP) for at least a week (24). It is thought to aid hole closure due
to the buoyant force of intraocular gas bubble [40]. The gas bubble keeps the
edges of macular hole dry and provides scaffold for glial cell proliferation
[38,41]. Also surface tension is constant around the bubble’s interface with
the retina as long as volume of gas is 2/3rd to 3/4th of
vitreous cavity [42].
But successful hole closure without FDP as
reported in Tornamby Pilot study supports the approach avoiding FDP [43].
Various other studies favored no FDP or a minimum of one day FDP and showed 90%
anatomical and functional success rate [44,45].
Endotamponading agents
In original description of macular hole
surgery, use of non- expansible gas of SF6 with 1 week FDP was indicated [23].
In patients who require long-term tamponade, cannot maintain positioning or
have to travel by air, silicone oil can be opted. But its removal requires
another surgery and also anatomical closure is found to be only 65% as compared
with C3F8 gas which has 91% success rate [46]. C3F8 and densiron 68 share
advantage of having longer effect and both do not require positioning.
Densiron-68 is a mixture of silicone oil and amphiphilic perflurohexyloctane,
which facilitates better contact with the retina compared with standard
silicone oil [47]. Vitrectomy and air tamponade combined with 1-3 day facedown
positioning produced an excellent rate of macular hole closure [42,48].
Types
of macular hole closure
On the basis of postoperative OCT findings,
closed macular holes have been classified into two groups:
Type 1: MH is closed without foveal defect
of the neurosensory retina.
Type 2: Foveal defect of the neurosensory
retina persists postoperatively although the whole rim of the MH is attached to
the underlying RPE with flattening of the cuff [49].
Complications
of surgery
1. Swelling of the arcuate retinal nerve fiber
layer (SANFL): The SANFL does not appear to impact the final BCVA and can be
expected to disappear in about 3 months [50]. There are two hypotheses
regarding the cause of SANFL [51]. The first hypothesis is that surgical forceps
cause direct damage to the retina when grasping the ILM while the second is
that ILM peeling causes damage to the Müller cell endplates that are attached
to the ILM [51].
Dissociated optic nerve fiber layer (DONFL), which is similar to the
SANFL is observed as small, spindle-shaped splitting adjacent nerve fiber
bundles on SDOCT [52] Not all patients who undergo ILM peeling will present
with the DONFL postoperatively, and there have been no significant differences
observed between eyes with and those without the DONFL with respect to BCVA or
macular sensitivity. The reason for DONFL presentation is also unclear,
although some authors speculated that the DONFL is caused by irregularly
distributed Müller cells following ILM peeling in regions that show a higher
density of nerve fiber bundles in the RNFL.
2. Retinal breaks: The incidence of retinal
break formation during macular hole surgery is 5.5% [53].
3. Retinal detachment.
4. Gas cataract: The cataract progression
following macular hole surgery is found to be 64% within first year. Therefore
these days it is advocated to undergo combined macular hole and cataract
surgery. Lens extraction also allows a more complete Vitrectomy [54].
CONCLUSION
Macular
hole can be caused by several factors. Not all macular holes need surgical
intervention. OCT has redefined the macular hole and its prognostication. Also
with the invention of MIVS, surgical techniques and functional outcomes has
been improved dramatically.
1.
Gass
JD (1995) Reappraisal of biomicroscopic classification of stages of development
of a macular hole. Am J Opthalmol 119: 752-759.
2.
Sen P,
Bhargava A, Vijaya L, George R (2008) Prevalence of idiopathic macular hole in
adult rural and urban south Indian population. Clin Exp Ophthalmol 36: 257-260.
3.
Aaberg
TM (1970) Macular holes: A review. Surv Ophthalmol 15: 139-162.
4.
Brown
GC (1988) Macular hole following rhegmatogenous retinal detachment repair. Arch
Ophthalmol 106: 765-766.
5.
Cohen
SM, Gass JDM (1994) Macular hole following severe hypertensive retinopathy.
Arch Ophthalmol 112: 878-879.
6.
Flynn
HW (1994) Macular hole surgery in patients with proliferative diabetic
retinopathy. Arch Ophthalmol 112: 877-878.
7.
Wagner-Schuman
M, Dubis AM, Nordgren RN (2011) Race- and sex-related differences in retinal
thickness and foveal pit morphology. Invest Ophthalmol Vis Sci 52: 625-634.
8.
Bronstein
MA, Trempe CL, Freeman HM (1981) Fellow of eyes with macular holes. Am J
Ophthalmol 92: 757-7561.
9.
Haouchine
B, Massin P, Gaudric A (2001) Foveal pseudocyst as the first step in macular
hole formation: A prospective study by optical coherence tomography.
Ophthalmology 108: 15-22.
10.
Duker
JS, Kaiser PK, Binder S, de Smet MD, Gaudric, et al. (2013) The International
Vitreomacular Traction Study Group classification of vitreomacular adhesion,
traction and macular hole. Ophthalmology 120: 2611-2619.
11.
Witkin
AJ, Ko TH, Fujimoto JG, Schuman JS, Baumal CR, et al. (2006) Redefining
lamellar holes and the vitreomacular interface: An ultrahigh-resolution optical
coherence tomography study. Ophthalmology 113: 388-397.
12.
Ullrich
S, Haritoglou C, Gass C, Schaumberger M, Ulbig MW, et al. (2002) Macular hole
size as a prognostic factor in macular hole surgery Br J Ophthalmol 86:
390-393.
13.
Schmitz-Valckenberg
S, Fleckenstein M, Scholl HP, Holz FG (2009) Fundus auto fluorescence and
progression of age-related macular degeneration. Surv Ophthalmol 54: 96-117.
14.
Kakehashi
A, Schepens CL, Trempe CL (1996) Vitreomacular observations II. Data on the
pathogenesis of idiopathic macular breaks. Graefes Arch Clin Exp Ophthalmol
234: 425-433.
15.
Smiddy
WE, Flynn HW Jr (2004) Pathogenesis of macular holes and therapeutic
implications. Am J Ophthalmol 137: 525-537.
16.
Green
WR (2006) The macular hole. Histopathological studies. Arch Ophthalmol 124:
317-321.
17.
Michalewska
Z, Michalewski J, Sikorski BL, Kałuzny JJ, Wojtkowski M, et al. (2009) A study
of macular hole formation by serial spectral optical coherence tomography Clin
Exp Ophthalmol 37: 373-383.
18.
Ezra E
(2001) Idiopathic full thickness macular hole: Natural history and
pathogenesis. Br J Ophthalmol 85: 102-109.
19.
Chen
JC, Lee LR (2008) Clinical spectrum of lamellar macular defects including
pseudo holes and pseudo cysts defined by optical coherence tomography. Br J
Ophthalmol 92: 1342-1346.
20.
Allen
AW, Gass JD (1976) Contraction of a perifoveal epiretinal membrane simulating a
macular hole. Am J Ophthalmol 82: 684-689.
21.
Haouchine
B, Massin P, Tadayoni R, Erginay A, Gaudric A (2004) Diagnosis of macular
pseudoholes and lamellar macular holes by optical coherence tomography. Am J
Ophthalmol 138: 732-739.
22.
Gass
JDM (1997) Vitreofoveal separation and lamellar hole formation. In: Gass JDM,
editor. Stereoscopic atlas of macular diseases: diagnosis and treatment. 4th
Edn. St Louis: CV Mosby, pp: 926-927.
23.
Margherio
RR, Schepens CL (1972) Macular holes II. Management. Am J Ophthalmol 74:
233-240.
24.
Kelly
NE, Wendel RT (1991) Vitreous surgery for idiopathic macular holes. Arch
Ophthalmol 109: 654-659.
25.
Min
WK, Lee JH, Ham DI (1999) Macular hole surgery in conjunction with endolaser
photocoagulation. Am J Ophthalmol 127: 306-311.
26.
Schocket
SS, Lakhanpal V, Miao XP (1987) Treatment of macular holes with the argon
laser. Trans Am Ophthalmol Soc 85: 159-175.
27.
Freeman
WR, Azen SP, Kim JW, el-Haig W, Mishell DR, et al. (1997) Vitrectomy for the
treatment of full-thickness stage 3 or 4 macular holes - Results of a
multicentred randomized clinical trial. Arch Ophthalmol 115: 11-21.
28.
Smiddy
WE, Feuer W, Cordahi G (2001) Internal limiting membrane peeling in macular
hole surgery. Ophthalmology 108: 1471-1478.
29.
Brooks
HL Jr (2000) Macular hole surgery with and without internal limiting membrane
peeling. Ophthalmology 107: 1939-1948.
30.
Rizzo
S, Belting C, Creasti F, Genovesi-Ebert F (2007) Sutureless 25-gauge vitrectomy
for idiopathic macular hole repair. Graefes Arch Clin Exp Ophthalmol 245:
1437-1440.
31.
Shimada
H, Nakashizuka H, Hattori T, Mori R, Mizutani Y, et al. (2009) Double staining
with brilliant blue G and double peeling for epiretinal membranes.
Ophthalmology 116: 1370-1376.
32.
Almony
A, Nudleman E, Shah GK, Blinder KJ, Eliott DB, et al. (2012) Techniques,
rationale and outcomes of internal limiting membrane peeling. Retina 32:
877-891.
33.
Shimada
N, Sugamoto Y, Ogawa M, Takase H, Ohno-Matsui K (2012) Fovea-sparing internal
limiting membrane peeling for myopic traction maculopathy. Am J Ophthalmol 154:
693-701.
34.
Morizane
Y, Shiraga F, Kimura S, Hosokawa M, Shiode Y, et al. (2014) Autologous
transplantation of the internal limiting membrane for refractory macular holes.
Am J Ophthalmol 157: 861-869.e1.
35.
Michalewska
Z, Michalewski J, Adelman RA, Nawrocki J (2010) Inverted internal limiting
membrane flap technique for large macular holes. Ophthalmology 117: 2018-2025.
36.
Shin
MK, Park KH, Park SW, Byon IS, Lee JE, et al. (2014)
Perfluoro-n-octane-assisted single-layered inverted internal limiting membrane
flap technique for macular hole surgery. Retina 34: 1905-1910.
37.
Aurora
A, Seth A, Sanduja N (2017) Cabbage leaf inverted flap ILM peeling for macular
hole: A novel technique. Ophthalmic Surg Lasers Imaging Retina 48: 830-832.
38.
Grewal
DS, Mahmoud TH (2016) Autologous neurosensory retinal free flap for closure of
refractory myopic macular holes. JAMA Ophthalmol 134: 229-230.
39.
Kumagai
K, Furukawa M, Ogino N, Uemura A, Demizu S, et al. (2004) Vitreous surgery with
and without internal limiting membrane peeling for macular hole repair. Retina
24: 721-727.
40.
Isomae
T, Sato Y, Shimada H (2002) Shortening the duration of prone positioning
aftermacular hole surgery- Comparison between 1 week and 1 day prone
positioning. Jpn J Ophthalmol 46: 84-88.
41.
Dhawahir-Scala
FE, Maino A, Saha K, Mokashi AA, McLauchlan R, et al. (2008) To posture or not
to posture after macular hole surgery. Retina 28: 60-65.
42.
Thompson
JT, Smiddy WE, Glaser BM, Sjaarda RN, Flynn HW Jr (1996) Intraocular tamponade
duration and success of macular hole surgery. Retina 16: 373-382.
43.
Tornambe
PE, Poliner LS, Grote K (1997) Macular hole surgery without face-down
posturing. A pilot study. Retina 17: 179-185.
44.
Madreperla
SA, Geiger GL, Funata M, de la Cruz Z, Green WR (1994) Clinicopathologic
correlation of macular hole treated by cortical vitreous peeling and gas
tamponade. Ophthalmology 101: 682-686.
45.
Simcock
PR, Scalia S (2018) Phacovitrectomy without prone posture for full thickness
macular holes. Br J Ophthalmol; 85: 1316-1319.
46.
Lai
JC, Stinnett SS, McCuen BW (2003) Comparison of silicone oil versus gas
tamponade in the treatment of idiopathic full thickness macular hole.
Ophthalmology 110: 1170-1174.
47.
Lappas
A, Heinrich Foerster AM, Kirchhof B (2009) Use of heavy silicone oil
Densiron-68 in the treatment of persistent macular holes. Acta Ophthalmol
87: 866-870.
48.
Eckardt
C, Eckert T, Eckardt U, Porkert U, Gesser C (2008) Macular hole surgery with
air tamponade and optical coherence tomography-based duration of facedown
positioning. Retina 28: 1087-1096.
49.
Kang
SW, Ahn K, Ham DI (2003) Types of macular hole closure and their clinical
implications. Br J Ophthalmol 87: 1015-1019.
50.
Pichi
F, Lembo A, Morara M, Veronese C, Alkabes M, et al. (2014) Early and late inner
retinal changes after inner limiting membrane peeling. Int Ophthalmol 34:
437-446.
51.
Clark
A, Balducci N, Pichi F (2012) Swelling of the arcuate nerve fiber layer after
internal limiting membrane peeling. Retina 32: 1608-1613.
52.
Spaide
RF (2102) Dissociated optic nerve fiber layer appearance after internal limiting
membrane removal is inner retinal dimpling. Retina 32: 1719-1726.
53.
Sjaarda
RN, Glaser BM, Thompson JT (1995) Distribution of iatrogenic retinal break in
macular hole surgery. Ophthalmology 102: 1387-1392.
54.
Duker
JS, Wendel R, Patel AC (1994) Late re-opening of macular holes after initially
successful treatment with vitreous surgery. Ophthalmology 101: 1373-1378.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Renal Transplantation Science (ISSN:2640-0847)