2811
Views & Citations1811
Likes & Shares
Intervertebral disc regeneration based on stem cell
differentiation is an attractive approach towards repairing/regenerating the
nucleus pulposus. Here, we attempted to repair degenerative intervertebral
discs using human umbilical cord mesenchymal stem cells (hUCMSCs) in
combination with type I collagen. The disc degeneration model was established
in New Zealand white rabbits by disc puncture. Two weeks after puncture,
rabbits showed typical intervertebral disc degeneration, with internal disc
disruption; stenosis of the intervertebral space, weakening T2 disc signal, and
decreased disc height. Using X-ray and T2-weighted MRI analyses, we
demonstrated that transplantation of hUCMSCs embedded in collagen type I
hydrogel exhibited a therapeutic effect in repairing degenerative discs, as
shown by better disc height index, lower disc degeneration grade, and
relatively preserved inner annulus structure with minimal fibrosis in the
nucleus region. Similarly, immunohistochemical and spectrophotometry analyses
revealed lower intervertebral disc degeneration grading, more nucleus pulposus cells,
higher expression of type II collagen and proteoglycan around nucleus pulposus
cells in the hUCMSC/collagen treated group. Collectively, our study
demonstrates co-transplantation of hUCMSCs and type I collagen has a
restorative potential in the treatment of intervertebral disc degeneration.
Keywords:
Intervertebral disc degeneration, Tissue engineering repair, Human
umbilical cord, Mesenchymal stem cells, Type I collagen, Rabbit
INTRODUCTION
Currently, strategies to regenerate the disc have focused primarily on
restoring the ability to restore the disc tissue. These include strategies
involving cell transplantation therapy, cytokine and growth factor induction,
gene therapy, and tissue engineering [9-13]. Multiple sources of donor cells
have been used for cell therapy to repair the degenerating intervertebral disc
(IVD). Autologous NP cell transplantation has become one of the major
techniques to prevent IVD in animal models [13,14]. However, it has been
considered clinically difficult for broad application as the procedure requires
more cells than can be harvested from a single disc. Some reports have
demonstrated that transplantation of bone marrow mesenchymal stem cells (MSCs)
delayed degeneration of the nucleus pulposus [15]. MSCs can be isolated from
tissues other than bone marrow [16-18]. Some studies have harvested potent
mesenchymal stem cell population from the Wharton’s jelly of the human
umbilical cord, which possess cell markers of multipotent mesenchymal stromal
cells and had ability to differentiate to osteogenic, adipogenic and chondrogenic
lineages in vitro under defined monolayer or cell mass-based differentiation
condition [19,20]. Leckie et al have presented data showing that injecting
human umbilical tissue-derived cells into the NP improved the biomechanical
properties of the degenerating IVD in vivo [21]. Wang et al showed that the
chondrogenic differentiation of hUCMSCs produced more glycosaminoglycan and
collagen than bone marrow MSCs, suggesting that hUCMSCs possesses a greater
potential for cell-based treatment of IDD [22]. Several studies have focused on
the use of hUCMSCs because of its potent repairing effect on degenerative
diseases and damaged organs [23,24]. Experimental hUCMSCs transplantation
therapies are effective in a variety of diseases including the articular cartilage
and myocardium [23,25]. The effect of hUCMSCs used in the nucleus pulposus
tissue engineering, however, has not well been fully explored.
Recently, tissue engineering using adult mesenchymal stem cells (MSCs)
as a candidate cell type has shown a great potential for cell-based treatment
of these spinal problems [26,27]. Many forms of biomaterials have been
investigated as scaffolds, such as alginate, chitosan, type I collagen, type II
collagen/aggrecan/hyaluronan, fibrin/hyaluronan and PLLA-hyaluronan nanofibres,
all of which serve as potential cell carriers for treatment of NP [28-32]. Type I collagen, a natural and frequently-used scaffold
material with special biological activity and biocompatibility, is
non-immunogenic, biodegradable and can withstand the mechanically loaded
environment in the IVD. This scaffold is degraded slowly to allow the seeded
cells to differentiate and produce new matrix [23,33]. Bertolo et al. showed
that MSCs in collagen matrixes produced more mRNA and proteins of the chondrogenic
markers collagen type I, collagen type II (COL2) and aggrecan (ACAN) compared
with cells embedded in alginate or chitosan [23]. Proteoglycan accumulation and
cell survival were also higher in collagen and gelatin matrixes. Type I
collagen is naturally present in the disc without immune rejection and
compatibility issues, so it may be a good scaffold material used for NP tissue
engineering. It has been shown that transplantation of BM-MSCs embedded in type
I collagen into articular cartilage defects improves arthroscopic and
histological grading scores. Kuroda et al transplanted collagen gel-embedded
BM-MSCs in an athlete with a grade IV cartilage defect and found that the
defect was covered with smooth tissues after seven months [26]. Wakitani et al
also found that transplanted type I collagen gel embedded autologous BM-MSCs
repaired cartilage defects with a fibrocartilage-like tissue after one year
[27]. These results suggest that type I collagen is a good scaffold for NP
tissue engineering.
Although MSCs used for tissue engineering have shown a great potential
in cell-based treatment of degenerative diseases, studies that combine UCMSCs
with type I collagen scaffold for NP tissue engineering have not well been
reported. In this study, we transplanted type I collagen scaffold-embedded hUCMSCs into degenerative intervertebral discs
of rabbit model, and assessed its therapeutic effects using radiography,
magnetic resonance imaging (MRI), and immunohistochemistry.
MATERIALS
AND METHODS
Isolation and characterization
of hUCMSCs
hUCMSCs were supplied by Shenzhen Beike Stem cell Engineering
Institute. The passages 2-3 of hUCMSCs were used for this study. The
immunophenotype of the culture-expanded hUCMSCs was analyzed by flow cytometry
for specific cell surface markers (CD90, CD73 and CD105), hematopoietic cell
markers (CD45, CD34, CD14 and CD19) and major histocompatibility elements
(HLA-DR). Flow cytometry was performed with the use of FACSCalibur. All
antibodies were purchased from BD Biosciences, CA.
Adipogenic, osteogenic and chondrogenic
differentiation
The multipotent ability of hUCMSCs at passage 2 was performed by
adipogenic, osteogenic and chondrogenic differentiation as previously described
previously [34,35]. After induction, cells were stained with the oil red O (Sigma, USA), alizarin red (Sigma, USA) or Alcian Blue (Sigma, USA) to detect the presence of
neutral lipid vacuoles in differentiated adipocytes, calcium deposition in
osteocytes or proteoglycan in chondrocyte, respectively.
Hydrogel preparation and chondrogenic
differentiation
Due to its non-immunogenic and biodegradable feature, we used type I
collagen as the supportive material in NP tissue engineering as previously
described [36]. Type I collagen was purchased from Shengyou Biotechnology Co.
(Hangzhou, China) and dissolved in 0.1% acetic acid with a concentration of 5
mg/mL. For the fabrication of collagen hydrogel, cells were suspended in
chondrogenesis-induced medium (high glucose DMEM, 0.1 µmol/L dexamethasone, 50
mg/L ascorbate, 1 mmol/L sodium pyruvate, 40 mg/L L-proline, 6.25
mg/L insulin, 6.25 mg/L transferrin,6.25
mg/L sodium selenite, 10 µg/L TGF-β1). Immediately before use, 600µl
collagen solution was neutralized with 40 µl neutralization buffer consisting
of 10x PBS and 0.1M NaOH. The neutralizing hydrogels (1.5, 2.5, and 3.5 mg/ml)
were gently mixed with hUCMSCs (7.5, 22.5, 67.5×105 cells/ml,
respectively), following the orthogonal experiment table design. The culture
medium/type I collagen scaffold constructs were used as the control.
All components were gently mixed to avoid air bubbles, and then seeded
into 96-wells plate. The plates with hydrogel were incubated at 37 ℃ for 15 min in a 5% CO2 humidified atmosphere for gel
polymerization. After polymerization, 150 μl chondrogenesis-induced medium were
added to the plate. Chondrogenic differentiation of hydrogel-embedded hUCMSCs
was further examined by histochemistry and immunohistochemistry staining for
the expression of type II collagen and GAG using primary mouse anti-type II
collagen antibody, rabbit anti-GAG antibody,
and an UltraSensitiveTM SP (Mouse/Rabbit) IHC Kit (MaiXin
BIO, Fuzhou, China), according to the manufacturer's protocol.
In vivo transplantation
Thirty-six New Zealand white rabbits, weighing an average of 2.5 kg,
were anesthetized with pentobarbital sodium (1 ml/kg). The rabbits were then
placed into a lateral prone position, and the anterior surface of the lumbar
spine was exposed through the anterolateral approach, and intervertebral discs
(L3 to L4, L4 to L5, and L5 to L6) were identified. Using a number 20-gauge, a
5 mm deep puncture was made into 3 contiguous discs (L3 to L4, L4 to L5, and L5
to L6) through the ventral anulus. Care was taken to avoid excessive exposure
of surrounding ligaments and tissues to avoid postoperative spur formation. The
disc L6 to L7 intact was used as a normal control. The wound was then
thoroughly irrigated with sterile saline and closed with layered sutures. The
rabbits were injected with penicillin 3 days after surgery and returned to
their cages after a short recovery observation. Two weeks post surgery, disc
degeneration was examined with MRI. Rabbits with IDD were randomly divided into
3 groups (12 per group): Control group (CG), Experimental group (EG), and
Degeneration group (DG). Animals were treated with collagen type I in the CG
group and hUCMSCs/type I collagen hydrogel in the EG group. Animals in the DG
group did not receive any treatment as the control. The discs that were not
punctured were collected as the normal group (NG). At 4, 8, 12 weeks
postoperatively, the pathological changes were evaluated by MRI, X-ray and
histological analysis.
Radiographic and MRI analysis
Rabbits were anesthetized with pentobarbital sodium (1 ml/kg).
Radiographs were taken using X-ray equipment (55KV,100mA,50ms).
The IVD height was expressed as the disc height index (DHI) based on the method
of Masuda et al. [37]. The average IVD height (DHI) was calculated by averaging
the measurements obtained from the anterior, middle, and posterior portions of
the IVD and dividing that by the average of adjacent vertebral body heights.
Alterations in the DHI of injected discs were expressed as %DHI and normalized to
the measured preoperative IVD height (%DHI = postoperative DHI/preoperative DHI
× 100).
MR imaging were taken using a Semiens 1.5-T imager. T2-weighted turbo
spin-echo images (TE 150 ms, TR 4,300 ms) of the lumbar spine were obtained at
each time point. MRI images of each disc section were graded according to the
method used by Pfirrmann et al. [38].
Histology and immunohistochemistry
The intervertebral discs including the adjacent vertebral bodies were
fixed in 10% neutral-buffered formalin and embedded in paraffin. Midline
sagittal sections of the intervertebral discs were stained with Hematoxylin and
Eosin. Based on the condition of anulus fibrosus, the border between the anulus
fibrosus and nucleus pulposus, the cellularity of the nucleus pulposus, and the
matrix of the nucleus pulposus through midsagittal sections, we graded each
disc section according to the histological grading scale developed by Masuda et
al. [37]. Cells randomly selected from four horizons in each slice were counted
under high magnification (× 400).
Proteoglycan
analysis
NP tissues of rabbits were collected and examined by phloroglucinol
spectrophotometry analysis for the expression of proteoglycan according to the
manufacturer's protocol.
Statistical
analysis
Data were expressed as the means ± SD. Statistical analysis was
performed by SPSS 17.0 software. The Student’s t-test was used to compare serum
parameters. P < 0.05 was
considered to indicate a statistically significant result.
RESULTS
Characterization of hUCMSCs
To verify the lineage potential, hUMSCs were differentiated in six-well
plates. After 21, 28 and 14 days of culture, cells were examined for
adipogenic, osteogenic and chondrogenic differentiation. Intracytoplasmic lipid
droplets stained with oil red O, calcium deposits stained with alizarin red and
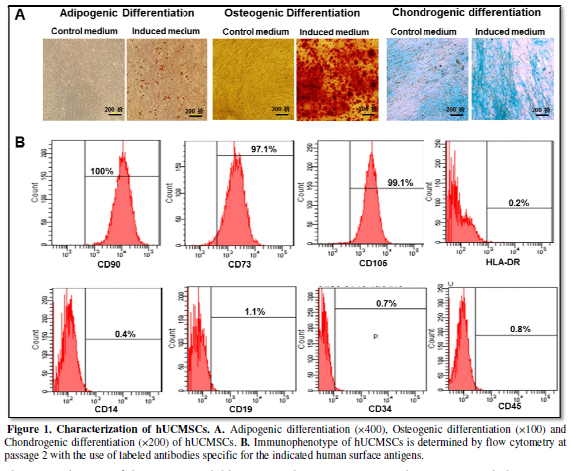
proteoglycan stained with Alcian Blue were observed in cell (Figure 1A), demonstrating the potential
of adipogenic, osteogenic and chondrogenic differentiation of the isolated
hUCMSCs.
The immunophenotype of the culture-expanded hUCMSCs at passage 2 was
analyzed by flow cytometry for specific cell surface markers. As shown in Figure 1B, hUMSCs were positive for
known MSC markers (CD105, CD73 and CD90) and were negative for hematopoietic
markers (CD34, CD45, CD19, CD14 and HLA-DR). After characterization, the
hUCMSCs were used for the subsequent study.
Immunohistochemical analyses of
chondrogenesis in type I collagen hydrogel
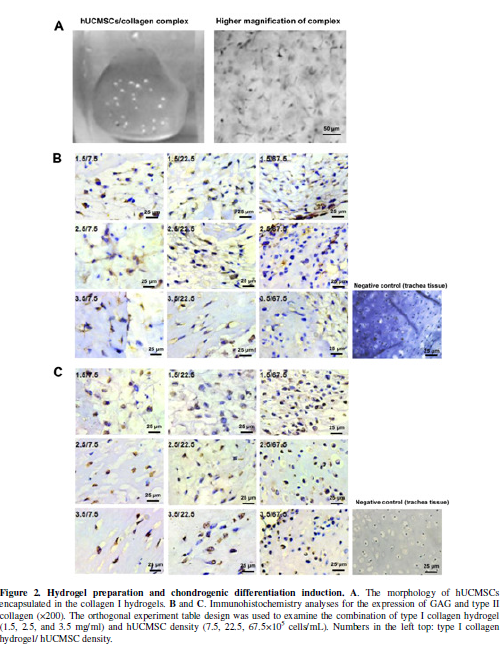
To develop nucleus pulposus-like tissue, variable amounts of hUCMSCs were seeded in scaffold consisting of three levels of type I collagen (1.5, 2.5, and 3.5 mg/mL). The culture medium/type I collagen scaffold constructs were used as the control. After 24 h of cell seeding, the morphology of hUCMSCs encapsulated in collagen I hydrogels were observed. As shown in Figure 2A, the hUCMSCs displayed the typical fibroblast-like morphology. After 2 weeks of culture in standard chondrocyte conditioning medium, the complex of hUCMSCs with type I collagen scaffolds was analyzed by immunohistochemistry analyses. We found that hUCMSCs embedded in collagen I hydrogel highly expressed GAG and collagen II after exposed cells-embedded hydrogels (Figure 2B, 2C). Among the 3x3 experiment design groups, the 7.5×105/ml hUMSCs/1.5mg/ml typeⅠ collagen group secreted the highest GAG and type II collagen. These data suggest that the collagen hydrogel embedded-MSCs were able to undergo chondrogenic differentiation and surrounded by sulfated proteoglycan-rich extracellular matrix.
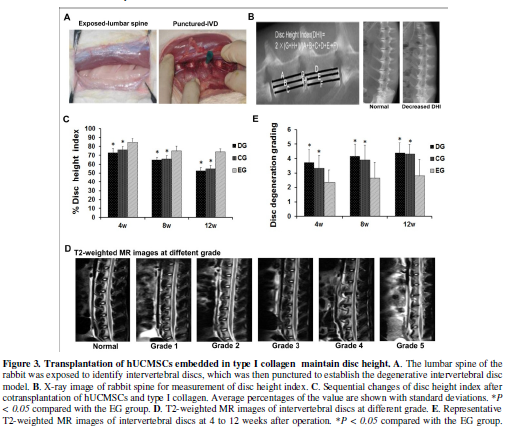
Imaging analyses of degenerative discs
To determine the effect of the hUCMSCs/type I collagen therapy on
repairing the degenerative discs, 36 New Zealand white rabbits were punctured
to establish animal model of degenerative intervertebral disc (Figure 3A). Twenty-four rabbits were
treated with culture medium/collagen type I (Control group, CG) and
hUCMSCs/type I collagen (Experimental group, EG), and another 12 rabbits were
untreated as the “degeneration group” (DG). The animals with discs un-punctured
were referred to the “normal group (NG)”. Two weeks post puncture, the changes
were evaluated by X-ray and MRI analysis. The IVD height was expressed as the
disc height index (DHI) based on the method of Masuda et al [37] (Figure 3B). Animals showed typical
internal disc disruption, including stenosis of the intervertebral space,
weakening T2 disc signal and decreased disc height, suggesting that
degenerative intervertebral disc rabbit models were successfully established.
After cell transplantation for 4, 8 and 12 weeks, changes in the DHI of
injected discs were evaluated. The X-ray analysis showed that the disc height
index in the CG and DG group decreased gradually and reached bottom at 12
weeks. However, the % DHI in the treated EG group was statistically higher than
that in the CG and DG group at any time points (Figure 3C).
T2-weighted MR images showed that the signal intensity of nucleus
pulposus in the CG and DG group considerably decreased at 4 weeks and
thereafter (Figure 3D). Although the
intensity in the EG group also gradually reduced compared to that in the NC
group, it remained higher than those in the CG and DG groups. The disc
degeneration grading of the EG group discs was graded as 2-3 at the most,
compared with 4-5 in the CG and DG groups. NC group discs maintained grade 0
throughout the study (Figure 3E).
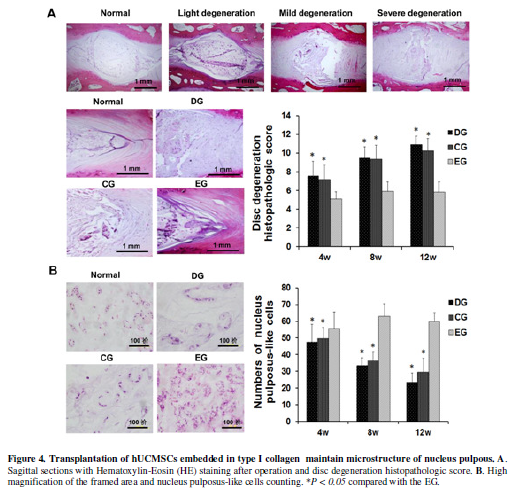
Transplantation of
hUCMSCs/type I collagen repairs the degenerative discs
The intervertebral discs including the adjacent vertebral bodies were
fixed in 10% neutral-buffered formalin and embedded in paraffin. Midline
sagittal sections of the intervertebral discs were stained with HE. Typical
histologic changes of degeneration were shown in Figure 4A. The discs in the EG group showed relatively preserved
inner annulus structure with minimal fibrosis in the nucleus region. The disc
degeneration histopathologic score in the EG group were lower than those in the
CG and DG groups. In the degeneration group, the nucleus pulposus could hardly
be seen. In the EG group, however, the nucleus pulposus looked comparable to
that in the normal group (Figure 4A).
In high magnified histology, the nucleus pulposus was replaced with fibrous
tissue in the CG and DG group but consisted of sparse cells surrounded with
matrix in the EG group. The number of nucleus pulposus-like cells in the EG
group were more than that in the CG and DG group (Figure 4B, p<0.05). Interestingly, the nucleus pulposus-like
cells was also relatively higher in the EG group than that in the normal group,
suggesting active regeneration in the degenerative intervertebral disc
following the treatment.
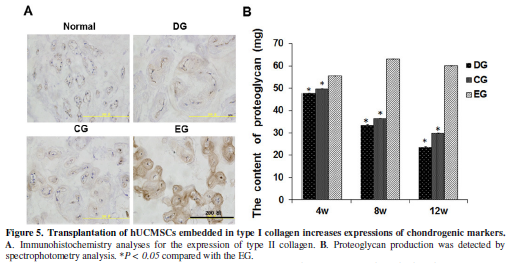
Immunohistochemistry analyses and proteoglycan assay revealed
significantly high expression of type II collagen and proteoglycan around
nucleus pulposus cells in the EG than that in the CG and DG groups (Figure 5A, 5B). These results indicate
that co-transplantation of hUCMSCs and type I collagen can restore the
extracellular matrix in degenerative discs.
DISCUSSION
Tissue engineering using adult mesenchymal stem cells (MSCs) has shown
a great potential for cell-based treatment of degenerative diseases and damaged
organs [26,27]. As an
alternative to MSCs, hUCMSCs possess clear advantages such as being obtained
from a readily available source as well as having low immunogenic potency and
high proliferative activity [19,20]. Several studies have reported the
therapeutic effects of hUCMSCs in degenerative full-thickness articular cartilage defects, myocardial infarction, and
osteogenesis imperfecta, bone regeneration, liver cirrhosis and acute
liver failure models [34,39,40]. Despite such an interest and the growing
number of research data, studies directed toward nucleus pulposus tissue
engineering to regeneration of IVD have not well been determined. Type I collagen is a protein-based three-dimensional
hydrophilic and polymeric networks with high water content facilitating rapid
diffusion of nutrients and metabolites. It allows embedded cells to grow in a
three-dimensional environment, which is very suitable for disc cells and
chondrocytes in vitro [23,33].
In this study, type I collagen was used here as the delivery scaffold to investigate the effect of
hUCMSCs on repairing the degenerative discs in a rabbits model.
To determine the effect of type I collagen-embedded hUCMSCs on
degenerative discs, hUCMSCs were isolated, cultured and evaluated in vitro for
osteogenic, adipogenic and chondrogenic differentiation potential and
immunophenotype. We showed that hUCMSCs were differentiated into osteogenic,
adipogenic and chondrogenic lineages and possess specific MSC cell surface
markers (CD105, CD73 and CD90), suggesting that the isolated cells possessed
the properties of MSC. The hUCMSCs were further seeded in collagen I hydrogel
to investigate their chondrogenic differentiation potential. Our data showed
that hUCMSCs embedded in collagen I hydrogel can undergo chondrogenesis
characterized by significantly increased expressions of GAG and collagen II,
the main collagenous (about 90% of the collagenous fraction) element within the
cartilage, suggesting that hUCMSCs undergo NP-like chondrogenesis in collagen I
scaffolds. Our result is consistent with that reported by Chen et al. [23], who
showed that hUCMSCs in type I collagen-hydrogel undergo chondrogenic
differentiation, indicating that hUCMSCs seeded in type I collagen scaffold was
suitable for nucleus pulposus tissue engineering. Thus, we used these hUCMSCs
embedded in collagen I for transplantation to investigate its role
in degenerative discs of rabbit model induced by puncture.
Restoration of disc height and T2-weighted signal intensity on MRI are
two major parameters for evaluating disc degeneration in clinical settings. A
high signal intensity of T2-weighted images in MRI is often used indirectly to
evaluate water content in the IVD [37,38]. Based on these parameters, the
degenerative intervertebral disc rabbit models we established showed typical
internal disc disruption; stenosis of the intervertebral space, weakening T2
disc signal and decreased disc height after two weeks of puncture, suggesting
that the degenerative intervertebral disc rabbit model was successfully
established. Degenerated IVDs were significantly improved according to X-ray
analyses after hUCMSCs-collagen I complex transplantation for 4 weeks. The DHI%
in the EG group remained higher than that in the CG and DG group at 4 weeks and
thereafter. T2 weighted MRI showed that the disc degeneration grading of EG
group discs were graded as 2-3 at the most, compared with 4-5 in the CG and DG
group. Immunohistological analyses revealed lower disc degeneration grading and
more nucleus pulposus cells in the EG than that of the CG and DG group at 4
weeks and thereafter.
Generally, type II collagen functions as a frame work in the nucleus
pulposus [41,42], maintaining disc height and histological features.
Proteoglycans are important components of the noncollagenous cartilage matrix
responsible for the mechanical properties of cartilage [43]. In our
experiments, the increase in the expression of cartilage ECM was detected by
immunohistochemical staining and spectrophotometry analysis. We showed higher
expression of type II collagen and proteoglycan around nucleus pulposus cells
in the EG group than that of the CG and DG group. Thus, these results indicate
that cotransplantation of hUCMSCs and type I collagen can restore the
extracellular matrix, which may be beneficial for the therapy of intervertebral
disc degeneration. Leckie et al have shown that injecting hUCMSCs into the NP
improved the biomechanical properties of the degenerating IVD in vivo [21].
Chen et al showed that hUCMSCs embedded in collagen hydrogel can undergo
chondrogenesis characterized by significantly increased expressions of
chondrogenic markers collagen II, aggrecan, COMP (cartilage oligomeric matrix
protein) and sox9 [23]. Taken together, the data indicate that transplantation
of hUCMSCs combined with type I collagen is a promising alternative approach in
nucleus pulposus tissue engineering.
In summary, our study has demonstrated that cotransplantation of
hUCMSCs and type I collagen exert a restorative effect in a degenerative
intervertebral disc rabbit model. Thus, chondrogenic differentiation of hUCMSCs
in type I collagen-hydrogel for nucleus pulposus tissue engineering may have a
potential application in the treatment of human IVD.
ACKNOWLEDGEMENTS
This study
was supported by Guangdong Natural
Science Foundation (2015A030313877, S2012010008531); Shenzhen Research
Grant (CXZZ20130516151903472, JCYJ20140411094549460 201302201); California
Institute of Regenerative Medicine (CIRM) grant (RT2-01942), the National
Natural Science Foundation of China grant (81272294, 31430021, 81372835) and
Jilin international collaboration grant (20130413010GH); and the grant of Key
Project of Chinese Ministry of Education (311015) and also Hu Jifan, NIH/NCI, 1
R43 CA 103553-01.
DISCLOSURE
The authors declare no conflict of interest.
- Katz
JN (2006) Lumbar disc disorders and low-back pain: socioeconomic factors
and consequences. J Bone Joint Surg Am 88: 21-24.
- Maniadakis
N, Gray A (2000) The economic burden of back pain in the UK. Pain 84:
95-103.
- Freemont
AJ (2009) The cellular pathobiology of the degenerate intervertebral disc
and discogenic back pain. Rheumatology (Oxford) 48: 5-10.
- Reuler
JB (1985) Low back pain. West J Med 143: 259-265.
- Deyo
RA, Weinstein JN (2001) Low back pain. New Eng J Med 344: 363-370.
- Mirza
SK, Deyo RA (2007) Systematic review of randomized trials comparing lumbar
fusion surgery to nonoperative care for treatment of chronic back pain.
Spine 32: 816-823.
- Hanley
EN, Herkowitz HN, Kirkpatrick JS, Wang JC, Chen MN, et al. (2010) Debating
the value of spine surgery. J Bone Joint Surg Am 92: 1293-1304.
- Ghiselli
G, Wang JC, Bhatia NN, Hsu WK, Dawson EG (2004) Adjacent segment
degeneration in the lumbar spine. J Bone Joint Surg Am 86: 1497-1503.
- Nishida
K, Kang JD, Suh JK, Robbins PD, Evans CH, et al. (1998)
Adenovirus-mediated gene transfer to nucleus pulposus cells. Implications
for the treatment of intervertebral disc degeneration. Spine 23:
2437-2442.
- Paul
R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, et al. (2003) Potential
use of Sox9 gene therapy for intervertebral degenerative disc disease.
Spine 28: 755-763.
- Takegami
K, Thonar EJ, An HS, Kamada H, Masuda K (2002) Osteogenic protein-1
enhances matrix replenishment by intervertebral disc cells previously
exposed to interleukin-1. Spine 27: 1318-1325.
- Gruber
HE, Johnson TL, Leslie K, Ingram JA, Martin D, et al. (2002) Autologous
intervertebral disc cell implantation: a model using Psammomys obesus, the
sand rat. Spine 27: 1626-1633.
- Ganey
T, Libera J, Moos V, Alasevic O, Fritsch KG, et al. (2003) Disc
chondrocyte transplantation in a canine model: a treatment for degenerated
or damaged intervertebral disc. Spine 28: 2609-2620.
- Okuma
M, Mochida J, Nishimura K, Sakabe K, Seiki K (2000) Reinsertion of
stimulated nucleus pulposus cells retards intervertebral disc
degeneration: an in vitro and in vivo experimental study. J Orthop Res18:
988-997.
- Wang
YH, Yang B, Li WL, Li JM (2015) Effect of the mixture of bone marrow
mesenchymal stromal cells and annulus fibrosus cells in repairing the
degenerative discs of rabbits. Gen Mol Res 14: 2365-2373.
- Sakaguchi
Y, Sekiya I, Yagishita K, Muneta T (2005) Comparison of human stem cells
derived from various mesenchymal tissues: superiority of synovium as a
cell source. Arthritis Rheum 52: 2521-2529.
- Mochizuki
T, Muneta T, Sakaguchi Y, Nimura A, Yokoyama A, et al. (2006) Higher
chondrogenic potential of fibrous synovium- and adipose synovium-derived
cells compared with subcutaneous fat-derived cells: distinguishing
properties of mesenchymal stem cells in humans. Arthritis Rheum 54:
843-853.
- Yoshimura
H, Muneta T, Nimura A, Yokoyama A, Koga H, et al. (2007) Comparison of rat
mesenchymal stem cells derived from bone marrow, synovium, periosteum,
adipose tissue, and muscle. Cell Tissue Res 327: 449-462.
- Weiss
ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, et al. (2008) Immune
properties of human umbilical cord Wharton's jelly-derived cells. Stem
Cells 26: 2865-2874.
- Cho
PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, et al. (2008) Immunogenicity
of umbilical cord tissue derived cells. Blood 111: 430-438.
- Leckie
SK, Sowa GA, Bechara BP, Hartman RA, Coelho JP, et al. (2013) Injection of
human umbilical tissue-derived cells into the nucleus pulposus alters the
course of intervertebral disc degeneration in vivo. Spine J 13: 263-272.
- Wang
L, Tran I, Seshareddy K, Weiss ML, Detamore MS (2009) A comparison of
human bone marrow-derived mesenchymal stem cells and human umbilical
cord-derived mesenchymal stromal cells for cartilage tissue engineering.
Tissue Eng 15: 2259-2266.
- Chen
X, Zhang F, He X, Xu Y, Yang Z, et al. (2013) Chondrogenic differentiation
of umbilical cord-derived mesenchymal stem cells in type I
collagen-hydrogel for cartilage engineering. Injury 44: 540-549.
- Okano
H (2002) Stem cell biology of the central nervous system. J Neurosci Res
69: 698-707.
- Sakai
D, Mochida J, Iwashina T, Hiyama A, Omi H, et al. (2006) Regenerative
effects of transplanting mesenchymal stem cells embedded in atelocollagen
to the degenerated intervertebral disc. Biomaterials 27: 335-345.
- Kuroda
R, Ishida K, Matsumoto T, Akisue T, Fujioka H, et al. (2007) Treatment of
a full-thickness articular cartilage defect in the femoral condyle of an
athlete with autologous bone-marrow stromal cells. Osteoarthritis
Cartilage 15: 226-231.
- [27]
Wakitani S, Imoto K, Yamamoto T, Saito M, Murata N, Yoneda M. Human
autologous culture expanded bone marrow mesenchymal cell transplantation
for repair of cartilage defects in osteoarthritic knees. Osteoarthritis
and cartilage / OARS, Osteoarthritis Research Society. 2002;10:199-206.
- [28]
Baer AE, Wang JY, Kraus VB, Setton LA. Collagen gene expression and
mechanical properties of intervertebral disc cell-alginate cultures.
Journal of orthopaedic research : official publication of the Orthopaedic
Research Society. 2001;19:2-10.
- Roughley
P, Hoemann C, DesRosiers E, Mwale F, Antoniou J, et al. (2006) The
potential of chitosan-based gels containing intervertebral disc cells for
nucleus pulposus supplementation. Biomaterials 27: 388-396.
- Halloran
DO, Grad S, Stoddart M, Dockery P, Alini M, et al. (2008) An injectable
cross-linked scaffold for nucleus pulposus regeneration. Biomaterials 29:
438-447.
- Stern
S, Lindenhayn K, Schultz O, Perka C (2000) Cultivation of porcine cells
from the nucleus pulposus in a fibrin/hyaluronic acid matrix. Acta
orthopaedica Scandinavica. 71: 496-502.
- Nesti
LJ, Li WJ, Shanti RM, Jiang YJ, Jackson W, et al. (2008) Intervertebral
disc tissue engineering using a novel hyaluronic acid-nanofibrous scaffold
(HANFS) amalgam. Tissue Eng 14:1527-1537.
- Bertolo
A, Mehr M, Aebli N, Baur M, Ferguson SJ, et al. (2012) Influence of
different commercial scaffolds on the in vitro differentiation of human
mesenchymal stem cells to nucleus pulposus-like cells. Eur Spine J 21:
S826-S838.
- Liu
Z, Meng F, Li C, Zhou X, Zeng X, et al. (2014) Human umbilical cord
mesenchymal stromal cells rescue mice from acetaminophen-induced acute
liver failure. Cytotherapy 16: 1207-1219.
- Chen
M, Zhang H, Wu J, Xu L, Xu D, et al. (2012) Promotion of the induction of
cell pluripotency through metabolic remodeling by thyroid hormone
triiodothyronine-activated PI3K/AKT signal pathway. Biomaterials 33:
5514-5523.
- Chen
X, Zhang F, He X, Xu Y, Yang Z, et al. (2013) Chondrogenic differentiation
of umbilical cord-derived mesenchymal stem cells in type I
collagen-hydrogel for cartilage engineering. Injury 44: 540-549.
- Masuda
K, Aota Y, Muehleman C, Imai Y, Okuma M, et al. (2005) A novel rabbit
model of mild, reproducible disc degeneration by an anulus needle
puncture: correlation between the degree of disc injury and radiological
and histological appearances of disc degeneration. Spine 30: 5-14.
- Pfirrmann
CW, Metzdorf A, Zanetti M, Hodler J, Boos N (2001) Magnetic resonance
classification of lumbar intervertebral disc degeneration. Spine 26:
1873-1878.
- Byeon
YE, Ryu HH, Park SS, Koyama Y, Kikuchi M, et al. (2010) Paracrine effect
of canine allogenic umbilical cord blood-derived mesenchymal stromal cells
mixed with beta-tricalcium phosphate on bone regeneration in ectopic
implantations. Cytotherapy 12: 626-636.
- Wang
J, Zhou X, Cui L, Yan L, Liang J, et al. (2010) The significance of CD14+
monocytes in peripheral blood stem cells for the treatment of rat liver
cirrhosis. Cytotherapy 12: 1022-1034.
- Humzah
MD, Soames RW (1988) Human intervertebral disc: structure and function.
Anat Rec 220: 337-356.
- Nerlich
AG, Boos N, Wiest I, Aebi M (1998) Immunolocalization of major
interstitial collagen types in human lumbar intervertebral discs of various
ages. Virchows Arch 432: 67-76.
- Quintana
L, zur Nieden NI, Semino CE (2009) Morphogenetic and regulatory mechanisms
during developmental chondrogenesis: new paradigms for cartilage tissue
engineering. Tissue Eng 15: 29-41.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)