2935
Views & Citations1935
Likes & Shares
Introduction:
With increasing
use of autologous fat grafting in the clinical setting, it is important
that techniques be refined in order to improve cosmetic outcomes, improve
volumetric retention, and reduce the need for repeat procedures. Here we
investigate the safety and efficacy of cell-assisted lipotransfer (CAL) using
uncultured stromal vascular fraction cells as a method to replace traditional
autologous fat grafting for facial applications.
Methods:
32 patients
received cell-assisted lipotransfer for a variety of indications including facial
aging, facial contour defects, and facial augmentation. Subjects were assessed
in terms of safety and tolerability as well as skin quality, patient
satisfaction, and volume restoration.
Results:
The mean volume
of cell-enhanced fat grafted was 23.5mL (range: 5-70 mL). The mean
volume of tissue submitted for point of care SVF cell isolation was 228.0 mL
(range: 50-700 mL). The average nucleated cell yield per gram of tissue
processed was 3.38x105 nucleated cells per gram of lipoaspirate
(1.10x105 - 7.0x105 nucleated cells/g) with an average
viability of 86.4% (67.2-97.8%). Overall, CAL to the face was well tolerated by
all the subjects. There were no instances of infection and no serious adverse
events reported. Only 1 patient out of 32 elected to undergo an additional
round of CAL.
Discussion:
The results
reported here demonstrate that CAL for facial indications can be done safely
at the point of care without introducing any additional risk to the subject and
can potentially reduce the need for additional procedures to achieve the
desired cosmetic result.
INTRODUCTION
In the
past 20 years, significant advances in the basic science of engraftment and
surgical technique have improved fat grafting outcomes and led to widespread
acceptance of autologous fat grafting as an option for a variety indications
requiring soft tissue augmentation and/or reconstruction. Adipose tissue is an
attractive tissue graft because it is easily acquired in large amounts with
liposuction techniques with little donor site morbidity. Autologous fat
grafting (AFG) of the face is now used for a wide variety of both
reconstructive and cosmetic indications including repair of congenital and post
traumatic defects, facial rejuvenation/augmentation and facial atrophy [1-3].
Facial fat grafting, as with other anatomic sites, is still
characterized by unpredictable rates of final engraftment volume frequently
requiring multiple graft procedures to achieve the desired clinical outcome.
One approach commonly used to combat this is over correction by injection of
excess fat graft, but over correction is typically unfeasible in the face since
the face can only accommodate small graft volumes. Another approach is
supplementation fat graft with stromal vascular fraction (SVF) cells, a method
termed cell-assisted lipotransfer (CAL) [4]. CAL is currently the most
promising strategy proposed to improve fat graft retention. Preclinical studies
show that CAL decreases the resorption rate of grafted adipose tissue and
results in improved final volume retention [4-6]. Unambiguous efficacy compared
to unenhanced fat grafting and dose-response curves have proven more difficult
to establish in the clinical setting but the supportive evidence is clearly
accumulating.
The SVF from adipose is a heterogeneous mixture of various nucleated
blood cells, preadipocytes, fibroblasts, smooth muscle cells, and both vascular
endothelial progenitors and adipose-derived stem cells [7-9]. These cells can
be quickly and safely isolated from excess lipoaspirate at the point of care
[10]. The adipose-derived stem cells (ASCs) contained within the stromal
vascular fraction have been shown to improve the permanent graft volume through
adipogenic and endothelial differentiation as well as through the production of
anti-inflammatory, antiapoptotic and proangiogenic cytokines which act in a
paracrine manner to maintain the viability of nearby adipocytes [11-14].
Because of volume limitations, fat grafting in the face is an excellent
opportunity to apply the promising strategy of CAL. In this paper we report on
the clinical results and safety in a retrospective, single center case series
review of 32 patients who underwent CAL to the face for a variety of
indications.
METHODS
A total of 32 subjects (3 male, 29 female) at Tower Outpatient Surgery
Center received cell-assisted lipotransfer to the face between September 2010
and September 2015. All patients provided written informed consent prior to
treatment under an IRB approved protocol. Subjects were treated for a variety
of indications including facial aging, facial contour defects, and facial
augmentation. Under IV sedation, subjects underwent infiltration with a
standard tumescent solution (lidocaine 1% with 1/100,000 epinephrine and
Marcaine 0.5% with 1/200,000 epinephrine) followed by suction-assisted
lipoplasty using a 3.0 mm cannula in order to harvest lipoaspirate. A portion
of lipoaspirate was set aside to decant for use as graft material and the
remaining volume was submitted to a trained technician in the operating room
for stromal vascular fraction cell separation.
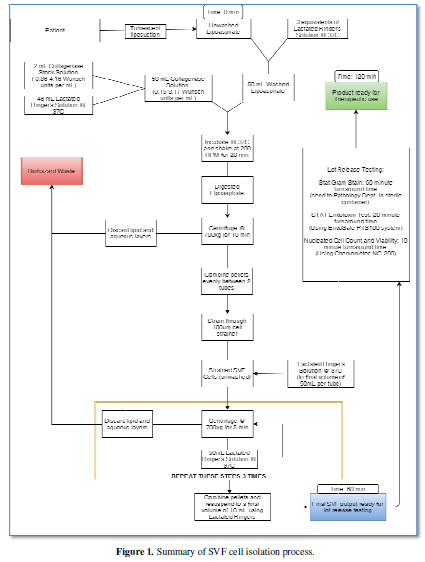
In order to isolate SVF cells (Figure
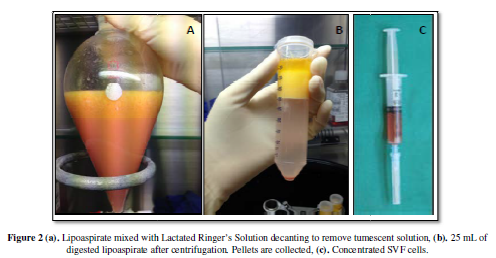
1), freshly harvested lipoaspirate is washed 3 times using an equivalent volume
of Lactated Ringer’s Solution (Figure 2a).
Washed lipoaspirate is aseptically aliquotted into sterile 50 mL conical tube
with collagenase solution in a 1:1 ratio. Final collagenase concentration
during digestion is 0.08 Wünsch units per mL (4 Wünsch units per 25 mL
lipoaspirate). Lipoaspirate is incubated with collagenase in a heated shaking
unit at 200 rpm for 20 minutes, inverting to mix every 5 minutes. Digested
lipoaspirate is then centrifuged for 10 minutes at 700xg. The fluid and lipid
layers are discarded (Figure 2b).
Cell pellets are concentrated and washed 3 times with Lactated Ringer’s
Solution. Washed cells are then strained through a 100um cell strainer in order
to remove detritus and other tissue fragments which may remain after washing.
Freshly isolated SVF cells (Figure 2c)
and graft material were homogenized and injected in a retrograde fashion using
a 19-gauge injection cannula into a grid covering the desired treatment area. A
portion of the isolated SVF cells were analyzed for nucleated cell counting and
viability, infection control (gram stain and aerobic culture), and bacterial
endotoxin testing.
RESULTS
The mean age of subjects was 56.3 years old (range: 28-75 years old)
with an average BMI of 22.2 kg/m2 (range: 17.1-27.1 kg/m2).
Subjects we followed for at least 6 months and as needed after that. Average
follow-up time was 8.6 months (range: 6-14 months). Table 1. summarizes the indications treated. The mean volume of
cell-enhanced fat grafted was 23.5mL (range: 5-70 mL). The mean volume of
tissue submitted for point of care SVF cell isolation was 228.0 mL (range:
50-700 mL). The mean total nucleated cell yield was 7.54x107 cells
(range: 6.6x106 - 2.5x108 nucleated cells). The average
nucleated cell yield per gram of tissue processed was 3.38x105
nucleated cells per gram of lipoaspirate (1.10x105 - 7.0x105
nucleated cells/g) with an average viability of 86.4% (67.2-97.8%). No bacteria
were seen on any gram strains. Bacterial endotoxin levels were below the
acceptable endotoxin limit in all cases. The mean time to isolate cells was 65
minutes (range: 60-90 min). Overall, CAL to the face was well tolerated by all
the subjects. There were no instances of infection and no serious adverse
events reported. Only 1 patient out of 32 elected to undergo an additional
round of CAL.
This case series also highlights the versatility of facial fat grafting
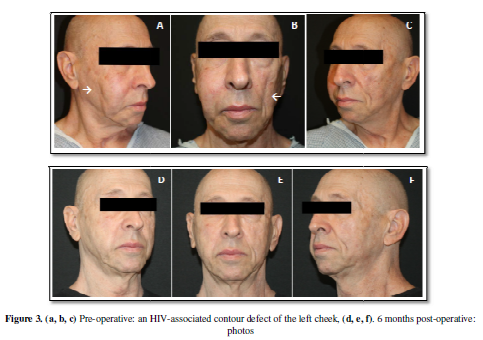
for a variety of indications. The subject pictured in Figure 3a, 3b, 3c is a 72 year old male who is HIV+. He presented
with an HIV associated contour deformity on the left cheek. Figure 3d, 3e, 3f show the results at 6
months. He did not require additional fat grafting and facial symmetry was
restored.
The subject shown in Figure 4 is a healthy, 75 year old female who
presented with typical aging of the face (Figure
4a, 4b, 4c). The subject received injections into the nasolabial fold and
surrounding areas. Again, the procedure was well tolerated and the subject did
not elect to receive any additional fat grafting. At 6 months (Figure 4d, 4e, 4f), we see an overall
improvement in the skin tone and quality as well as significant softening of
the age lines around the mouth and chin.
The subject shown in Figure 5 is a 57 year old male who presented with
acne scarring (Figure 5 a, 5b, 5c).
The subject received cell-enhanced fat injections throughout the affected
areas. The subject did not require additional treatment and as a result of
injections scarring was noticeably reduced and overall skin quality was
improved (Figure 5d, 5e, 5f).
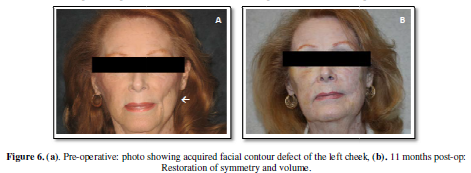
The subject shown in Figure 6 is a 74 year old female who presented
with a facial contour defect resulting from the excision of a facial malignancy
2 years prior (Figure 6a).
Using CAL, the defect was able to be filled and eliminated in a single
procedure (Figure 6b). There was no
recurrence of malignancy in the treated area.
DISCUSSION
With autologous fat grafting in the last 20 years, there still remains
a large amount of variability in the clinical outcomes due to a wide range of
factors including graft harvest and preparation techniques, volume of fat
transferred, injection technique, and patient to patient variability. With such
a large amount of variability, specifically in techniques used, there is no
clear consensus as to the best methods which should be employed. The major
deterrent of autologous fat grafting is that the final graft volume retention
is unpredictable and varies significantly with reports ranging from 10-90%
retention [15]. This unpredictability often leads to multiple treatment
sessions being required in order to achieve a satisfactory result [16]. This
can make the treatment very expensive if multiple sessions are required.
Improving the single session outcomes is beneficial because it can reduce the
overall cost to achieve the required results and ultimately make the procedure
available to a wider range of potential subjects. While more research is
required in order to find the optimal technique for fat grafting, supplementing
the graft material with autologous stromal vascular fraction cells
(cell-assisted lipotransfer) has been shown to increase the overall volume of
tissue which becomes permanently engrafted [3].
Several studies have been published demonstrating outcomes of
cell-assisted lipotransfer to the face. Schendel et al. (2015) examined the 3D
volumetric retention of cell-assisted lipotransfer to the face [3]. 12 subjects
received CAL to the face and were followed with facial scans to track 3D
volumetric retention over the course of a year (average follow-up 12.6 months).
Schendel et al. reported 68% mean volume retention at 12 months. Schendel et
al. drew comparison to another study by Gerth et al. (2014) which reported results
from 26 patients (mean follow-up 17 months) with 41.2% mean volume retention in
a study using similar methods, but using autologous fat grafting instead of
cell-assisted lipotransfer [17]. The comparison of the two supports the idea
that SVF-cell supplementation does improve the long-term volume retention.
Sterodimas et al. [16] compared results of AFG and CAL in a group of 20
subjects with congenital or acquired facial tissue defects. Of the 10 subjects
who received AFG alone, 7 required additional procedures in order to achieve
satisfactory results, whereas all 10 subjects in the CAL groups were satisfied
after only 1 procedure. Another study by Yoshimura et al. [2] compared clinical
outcomes of AFG and CAL in 6 subjects with facial lipoatrophy due to lupus
profundus or Parry-Romberg syndrome [18]. All subjects obtained improved facial
contour, but subjects in the CAL group (n=3) had better clinical improvement
reported overall. Overall, the general trend observed in clinical publications
of CAL to the face is that the use of CAL is superior to AFG alone in terms of
clinical outcome and can reduce the need for additional procedures.
CONCLUSION
The results reported here further demonstrate the applicability of CAL as a suitable substitute to conventional AFG in the clinical setting for facial applications. Only 1 patient desired additional treatment after receiving the initial treatment. Another important aspect of this case series is that the procedure was well tolerated and there were no adverse events reported in any of the 32 subjects treated. As SVF cell-based therapies begin to translate into the clinical setting, it is important to highlight the safety and tolerability of the procedures. The results reported here demonstrate that CAL for facial indications can be done safely at the point of care without introducing any additional risk to the subject compared to standard autologous fat grafting while negligibly increasing the operation time if concurrent procedures are being conducted.
- Charles
de Sa L, Gontijo de Amorim NF, Takiya CM, Borojevic R, Benati D, et al.
(2015) Antiaging treatment of the facial skin by fat graft and
adipose-derived stem cells. Plast
Reconstru Surg 135: 999-1009
- Yoshimura
K, Sato K, Aoi N, Kurita M, Inoue K, et al. (2008) Cell-assisted
lipotransfer for facial lipoatrophy: efficacy of clinical use of
adipose-derived stem cells. Dermatol
Surg 34: 1-8.
- Schendel
SA (2015) Enriched autologous facial fat grafts in aesthetic surgery: 3D
volumetric results. Aesthet Surg
J 35: 913-919.
- Matsumoto
D, Sato K, Gonda K, Takaki Y, Shigeura T, et al. (2006) Cell-assisted
lipotransfer: supportive use of human adipose-derived cells for soft
tissue augmentation with lipoinjection. Tissue Eng 12: 3375-3383.
- Garza
RM, Paik KJ, Chung MT, Duscher D, Gurtner GC, et al. (2014) Studies in fat
grafting: Part III. Fat grafting irradiated tissue: Improved skin quality
and decreased fat graft retention. Plast
Reconstr Surg 134:
249-257.
- Paik
KJ, Zielins ER, Atashroo DA, Maan ZN, Duscher D, et al. Studies in fat
grafting: Part V. Cell-assisted lipotransfer to enhance fat graft
retention is dose dependent. Plast
Reconstr Surg 136: 67-75.
- Bourin
P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, et al. (2013) Stromal
cells from the adipose tissue-derived stromal vascular fraction and
culture expanded adipose tissue-derived stromal/stem cells: a joint
statement of the International Federation for Adipose Therapeutics and
Science (IFATS) and the International Society for Cellular Therapy (ISCT).
Cytotherapy 15: 641-648.
- Gimble
JM, Bunnell BA, Chiu ES, Guilak F (2013) Concise review: adipose-derived
stromal vascular fraction cells and stem cells: let’s not get lost in
translation. Stem Cells
29: 749-754.
- Oedayraj
singh Varma MJ, Bruels RGM, Schouten TE, Jurgenset Wouter JFM, Bontkes HJ,
et al. (2007) Phenotypical and functional characterization of freshly
isolated adipose tissue-derived stem cells. Stem Cells and Dev
16: 91-104.
- Aronowitz
JA, Lockhart RA, Hakakian CS, Hicok KC (2015) Clinical safety of stromal
vascular fraction separation at the point of care. Ann Plast Surg 75: 666-671.
- Eto
H, Kato H, Suga H, Aoi N, Doi K, et al. (2012) The fate of adipocytes
after nonvascularized fat grafting: evidence of early death and
replacement of adipocytes. Plast
Reconstr Surg 129: 1081-1092.
- Planat-Benard
V, Silvestre JS, Cousin B, André M, Nibbelink M, et al. (2004) Plasticity
of human adipose lineage cells toward endothelial cells: physiological and
therapeutic perspectives. Circulation
109: 656–663.
- Naderi
N, Wilde C, Haque T, Francis W, Seifalian AM, et al. (2014) Adipogenic
differentiation of adipose-derived stem cells in 3-dimensional spheroid
cultures (microtissue): implications for the reconstructive surgeon. J Plast Reconstr Aesthet Surg
67: 1726–1734.
- Rehman
J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, et al. (2004)
Secretion of angiogenic and antiapoptotic factors by human adipose stromal
cells. Circulation 109:
1292-1298.
- Gir
P, Brown SA, Oni G, Kashefi N, Mojallal A, et al. (2012) Fat Grafting:
Evidence-based review on autologous fat harvesting, processing,
reinjection, and storage. Plast Reconstr Surg 130: 249-258.
- Sterodimas
A, de Faria J, Nicaretta B, Boriani F (2011) Autologous fat
transplantation versus adipose-derived stem cell-enriched lipografts: a
study. Aesthet Surg J 31:
682-693.
- Gerth
DJ, King B, Rabach L, Glasgold RA, Glasgold MJ (2014) Long-term volumetric
retention of autologous fat grafting processed with closed-membrane
filtration. Aesthet Surg J
34: 985-994.
- El-Kehdy
J, Abbas O, Rubeiz N (2012) A review of Parry-Romberg syndrome. J Am Acad Dermatol 67: 769-784.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Journal of Alcoholism Clinical Research