2903
Views & Citations1903
Likes & Shares
The
possibility and importance of paracrine effect of stem cell transplantation
were discussed in this commentary. Although the primary purpose of stem cell
transplantation therapy is absolutely the differentiation and incorporation of
engrafted cells to facilitate the target tissue reconstitution. However, the
paracrine effects surrounding recipient cells and/or donor cells each other is
also a reasonable issue. We have experienced a typical case of paracrine effect
in the experimental therapy of peripheral nerve injury using skeletal
muscle-derived stem cells (Sk-SCs). The severe nerve injury with long-gap was
made in the mouse and rat sciatic nerve, and bridged by a cellular conduit. The
mouse Sk-CSs and bone marrow stromal cells (BMSCs) were obtained from GFP-Tg
mice, and transplanted into the conduit. After 8-weeks, transplanted Sk-SCs
differentiated into all the peripheral nerve support cells (such as Schwann,
endoneurial/perineurial cells), and contributed to the recovery of the number
of axon for almost 90% and close to 60% of myelin following significant functional
recoveries. In contrast, BMSCs group showed the results similar to the non-cell
transplanted control, and cells were eliminated during the first week. On the
other hand, we also found that the human Sk-SCs, sorted as CD34+/45-
(Sk-34) cells, showed wholly comparable results of the mouse (such as cell
engraftment, differentiation, and recoveries of axon/myelin, and
functions)after 12-weeks of transplantation. However, the human CD34-/45-/29+
(Sk-DN) cells, which was composed mostly skeletal-myogenic cells, showed no
engraftment in the nerve tissue, but remained during 4 weeks, and showed
significantly higher numerical and functional recoveries than the control,
while these were clearly lower than Sk-34. This result suggested two important
points; 1)the skeletal-myogenic cells are not able to grow and differentiate in
the peripheral nerve specific niche, but 2) they accelerate nerve recovery,
probably their paracrine effects. It is likely that during 4-weeks of cell
survival with paracrine after transplantation is a sufficient condition, but
one-week is insufficient.
INTRODUCTION
Recently, we experienced the certain case of the paracrine effects in
the experimental therapies of the severely damaged peripheral nerve with a
log-gap, using mouse and human skeletal muscle-derived stem cells (Sk-SCs).
Because of this background, the paracrine effects of these cells before and
after transplantation are discussed in this commentary, with the comparison
with the case of bone-marrow-derived stromal cells (BMSCs).
Loss of vital functions in the somatic motor and sensory nervous
systems can be induced by severe peripheral nerve transection with a long gap
following trauma. In such cases, autologous nerve grafts have been used as the
gold standard, with the expectation of activation and proliferation of
graft-concomitant Schwann cells associated with their paracrine effects.
However, there are a limited number of suitable sites available for
harvesting of nerve autografts due to the unavoidable sacrifice of other
healthy functions. To
overcome this problem, we examined the potential of Sk-SCsas
a novel alternative cell source for peripheral nervere generation therapy [1,2].The
reason is that the Sk-SCs including CD34+/45- (Sk-34) [3] and CD34-/45- (Sk-DN)
[4] cells, which showed differentiation
potential into mesodermal cells (skeletal muscle cells, vascular smooth muscle
cells, pericytes and endothelial cells) and ectodermal cells (Schwann cells and
perineurial cells) in vivo, and typically exerted synchronized reconstitution
of the muscular, vascular and peripheral nervous system in the severely damaged
skeletal muscle[5-7]. The potential was
not changed, when both Sk-34 and Sk-DN cells were used by the mixed cells, as
the Sk-SCs [8-10]. Because of this
background, we made sciatic nerve long-gap transection model of mice (7-mm) and
rats (12-mm), and bridged using anacellular conduit made from separated
esophageal submucous membrane from mice after 3-days of 70% ethanol treatment.
Then, the cells were injected in the bridging-conduit [2]. As we expected in the mouse-to-mouse experiment, the
transplanted Sk-SCs differentiated into all the peripheral nerve support
cells(such as Schwann, endoneurial/perineurial cells), and contributed to the
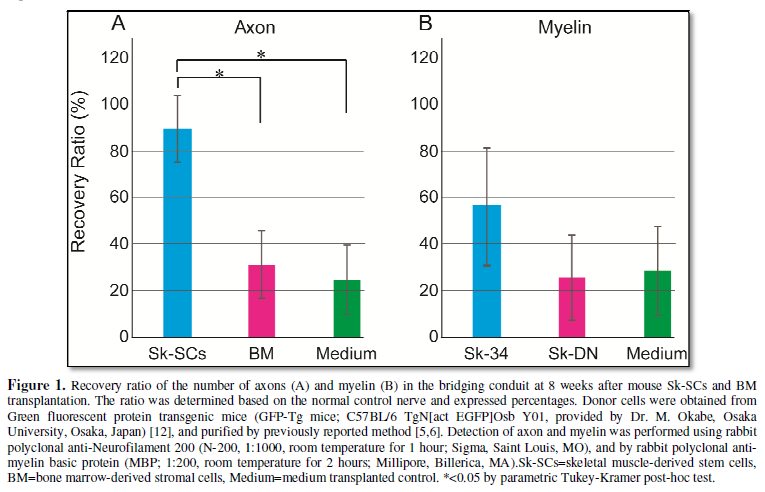
recovery of the number of axon for almost 90% and close to 60% of myelin (Figure 1A and 1B). Interestingly,
myogenic cells were also observed in this nerve niche, but the
skeletal myogenic capacity of expanded Sk-SCs had been diminished gradually, and completely
disappeared at 4-weeks after the transplantation [2]. Similarly, BMSCs transplantation was
also performed, however, the results showed almost no effects (wholly
comparable to the level of medium control) for the recoveries of the number of
axons and myelin (Figure 1A and B).
In addition, transplanted BMSCs disappeared during first one week. Therefore,
no cell engraftment with no effect is reasonable in this case.
We also established the appropriate
isolation and expansion culture method for the humanSk-34 and Sk-DN cells, and
transplanted them into the sever muscle injury model of nude mouse and rat [11]. Then, we found that the combined results
of both cell transplantation showed wholly comparable differentiation
capacities of the mouse Sk-SCs [11].
However, very interestingly, the human Sk-DN fraction showed the limited
inclusion of the skeletal-myogenic cells, whereas, the other multipotent stem
cells were contained in the Sk-34 fraction [11].
Therefore, we applied human Sk-34 and Sk-DN cells to the nerve-gap model separately,
because the elimination of skeletal-myogenic cells have been already confirmed
in the previous mouse Sk-SCs experiment [2].As
expected, the human Sk-34 cells showed cellular differentiations comparable to
the mouse Sk-SCs (data not shown), with favorable recovery of the number of
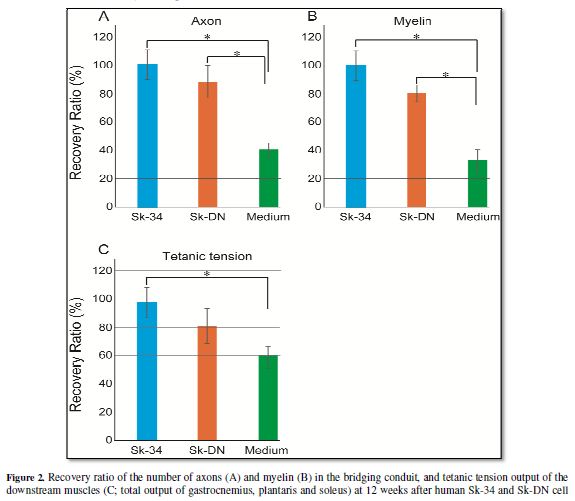
axons and myelin (Figure 2A and 2B),
and the contractile functions of downstream muscles (Figure 2C) at 12-weeks after transplantation. More interestingly,
human Sk-DN cells (skeletal myogenic cell dominant population) were also
eliminated similar to the above case of BMSCs, but the term took 4-weeks after
transplantation. A similar elimination of skeletal-myogenic cells in the nerve
niche were also confirmed in the previous mouse Sk-SCs during 4-weeks after transplantation
[2].Thus, it is certain that the
skeletal-myogenic cell is not able to growth and differentiates in the
peripheral nerve specific niche. In contrast, it is also clear that the Sk-DN
cells remained during 4-weeks after transplantation, whereas the BMSCs was
eliminated within one-week. In addition, the human Sk-DN group showed
significantly higher regenerations than that of the medium control at 12-weeks
after transplantation, while the levels are apparently lower than the Sk-34
group (Figure 2A, 2B and 2C).
The observed
differences in both groups are considered due to the prolonged survival/engraftment/paracrine
of Sk-34 cells and their differentiation into all the nerve support cells.
However, it is also certain that the complete elimination of the human Sk-DN
cells occurred during 4-weeks after transplantation, but significantly higher
morphological and functional recoveries than the control was achieved.
Therefore, this is reasonable to consider that this result may be due to the
paracrine effects of human Sk-DN cells during 4-weeks after transplantation.
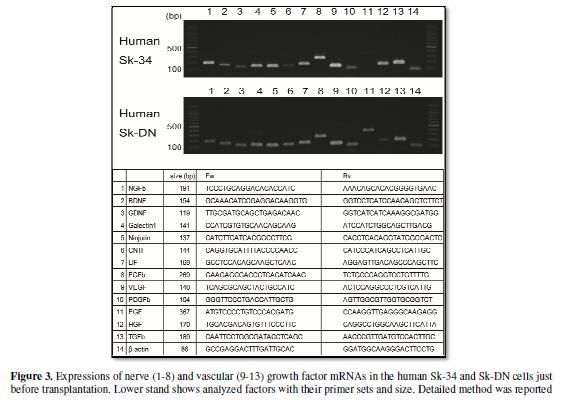
Putative paracrine capacities of human Sk-34 and Sk-DN cells, which was
supposed by expressions of mRNAs were shown in Figure 3. Both cells consistently expressed 8 analyzed nerves and 5
analyzed vascular growth factors just before the transplantation, and a
comparable paracrine effect can be expected. In fact, these expressions of
mRNAs have wholly kept in the Sk-34 cells even at 4-weeks after
transplantation; this was confirmed by the analysis for the engrafted Sk-34
cells, which was re-isolated enzymatically, and sorted using human-specific
antibody (data not shown). In the comparison of the above mouse and human cell
studies, both the mouse BMSCs and the human Sk-DN cells were eliminated in the
peripheral nerve niche, but the former showed no effects for nerve regeneration,
and the latter showed a significant contribution for the recovery. What makes a
difference between them; that is the term of elimination, thus how long stay in
the damaged tissue is considered to be the limiting factor of the paracrine
effects following stem cell transplantation, because the expressions of
nerve-vascular growth factor mRNAs were similar in both the mouse BMSCs [2] and
present human Sk-DN cells (Figure 3)
at just before the transplantation. In these view points, it is likely that one
week of the cell retaining-period is insufficient, but 4-weeks retaining, with
continuous expressions of growth factors after the onset of the damage, may
have a beneficial effect on the nerve regeneration. However, we still not
perform a more quantitative analysis (such as real-time RT-PCR) for the
expressions of nerve-vascular growth factors in Sk-34 and Sk-DN cells.
Therefore, further experiments, to clarify which factors may play a more
prominent paracrine role during regeneration process, should be necessary.
From the other point of view, it was also
suggested that the paracrine substances of skeletal-myogenic cells exert
facilitative effects for the peripheral nerve regeneration process. This
concept further suggests that the skeletal muscle fiber and peripheral nerve regeneration
process may share the large number of essential factors, and they co-working
together. This notion also supports the previous results of Sk-SCs
transplantation that asynchronized reconstitution of muscle-nerve-blood vessels
were induced in the severe muscle injury with a sizable defect [5,6].
Finally, the cytokine supply associate
with the stem cell transplantation was proposed in this commentary. However,
administration therapy of recombinant cytokines, which will be selected
appropriately in the near future, should be also considered. In particular, the
nerve injury categorized in the Seddons’s axonotmesis and/or the Sunderland’s
fourth degree, because the continuity of the epineurium (the most outer layer) is
maintained, but involves loss of axons, endoneurial tubes, perineurial
fasciculi and vascular networks, thus, associated with a bad prognosis. In
addition, a development of the nerve bridging materials, which enable a
sustained-release of cytokines, such as the biodegradable tubes, is also
much-needed.
CONCLUSION
Through these studies, it is supposed that the
first 2-3 weeks from the onset of nerve damage may be a critical period to
supply nerve-vascular growth factors to obtain the better nerve regeneration.
This notion is found to be useful for future cytokine therapy for the severe
peripheral nerve injury.
- Tamaki
T (2014) Bridging long gap peripheral nerve injury using skeletal
muscle-derived multipotent stem cells. Neural Regen Res 9: 1333-1336.
- Tamaki
T, Hirata M, Soeda S, Nakajima N, Saito K, et al. (2014) Preferential and
comprehensive reconstitution of severely damaged sciatic nerve using
murine skeletal muscle-derived multipotent stem cells. PLoS One 9: e91257.
- Tamaki
T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, et al. (2002).
Identification of myogenic-endothelial progenitor cells in the
interstitial spaces of skeletal muscle. J Cell Biol 157: 571-577.
- Tamaki
T, Akatsuka A, Okada Y, Uchiyama Y, Tono K, et al. (2008) Cardiomyocyte
formation by skeletal muscle-derived multi-myogenic stem cells after
transplantation into infarcted myocardium. PLoS ONE 3: e1789.
- Tamaki
T, Uchiyama Y, Okada Y, Ishikawa T, Sato M, et al. (2005) Functional
recovery of damaged skeletal muscle through synchronized vasculogenesis,
myogenesis, and neurogenesis by muscle-derived stem cells. Circulation
112: 2857-2866.
- Tamaki
T, Okada Y, Uchiyama Y, Tono K, Masuda M, et al. (2007) Synchronized
reconstitution of muscle fibers, peripheral nerves and blood vessels by
murine skeletal muscle-derived CD34(-)/45 (-) cells. Histochem Cell Biol
128: 349-360.
- Tamaki
T, Okada Y, Uchiyama Y, Tono K, Masuda M, et al. (2007) Clonal
multipotency of skeletal muscle-derived stem cells between mesodermal and
ectodermal lineage. Stem Cells 25: 2283-2290.
- Saito
K, Tamaki T, Hirata M, Hashimoto H, Nakazato K, et al. (2015)
Reconstruction of Multiple Facial Nerve Branches Using Skeletal
Muscle-Derived Multipotent Stem Cell Sheet-Pellet Transplantation. PLoS
One 10: e0138371.
- Soeda
S, Tamaki T, Hashimoto H, Saito K, Sakai A, et al. (2013) Functional
Nerve-Vascular Reconstitution of the Bladder-Wall; Application of Patch
Transplantation of Skeletal Muscle-Derived Multipotent Stem Cell
Sheet-Pellets. J Stem Cell Res Ther 3: 142.
- Tamaki
T, Soeda S, Hashimoto H, Saito K, Sakai A, et al. (2013) 3D reconstitution
of nerve-blood vessel networks using skeletal muscle-derived multipotent
stem cell sheet pellets. Regen Med 8: 437-451.
- Tamaki
T, Uchiyama Y, Hirata M, Hashimoto H, Nakajima N, et al. (2015)
Therapeutic isolation and expansion of human skeletal muscle-derived stem
cells for the use of muscle-nerve-blood vessel reconstitution. Front
Physiol 6: 165.
- Okabe
M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y (1997) 'Green mice' as a
source of ubiquitous green cells. FEBS Lett 407: 313-319.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- Dermatology Clinics and Research (ISSN:2380-5609)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Spine Diseases