2800
Views & Citations1800
Likes & Shares
Human induced pluripotent stem (hiPS) cells are defined as its ability
of self-renewal and pluripotency, and widely used for studies of
lineage-specific differentiation in vitro.
Under appropriate conditions, hiPS cells differentiate into hepatocyte-like cells,
recapitulating human embryonic development. Micro RNAs, short non-coding RNAs,
are important regulators for biological events via suppressing gene expression,
but little is known about how miRNAs modulate hepatic differentiation from
pluripotent hiPS cells. In this report, we focused on miR-27b which has been
reported to be upregulated during hepatic differentiation. To assess the role
of miR-27b, hiPS cell lines with the Doxycycline-inducible miR-27b expression
cassette integrated into Adeno-associated virus integration site (AAVS1) locus
were generated utilizing CRISPR/Cas9 genome editing technology and they were
subjected to the hepatic differentiation in
vitro. Induced expression of miR-27b in undifferentiated hiPS cells
repressed NANOG and OCT3/4, crucial genes for maintaining
self-renewal and pluripotency. During the definitive endoderm differentiation,
miR-27b-induction caused changes in expression of genes associated to
definitive endodermal and mesodermal lineage specific differentiation, depending
on the timing of induction, and consequently inhibited the hepatic
differentiation. These results demonstrated that proper suppression of miR-27b
expression in undifferentiated hiPS cells and during early stage of hepatic
differentiation is required to keep undifferentiated state of hiPS cells and to
secure correct differentiation of hepatocyte-like cells via endoderm formation.
Keywords
Human induced pluripotent stem cells, Hepatic differentiation, miR-27b,
Doxycycline-inducible expression
Abbreviation: DOX: Doxycycline;
AAVS1: Adeno-associated Virus Integration Site 1; αAT: alpha-1 Antitrypsin;
HNF: Hepatocyte Nuclear Factor; FGF: Fibroblast Growth Factor; FBS: Fetal
Bovine Serum; BMP: Bone Morphogenetic Protein; HCM: Hepatocyte Culture Medium; HGF:
Hepatocyte Growth Factor; OsM: Oncostatin M; BSA: Bovine Serum Albumin; FOXA2:
Forkhead Box A2; GSC: Goosecoid; MIXL1: Mix Paired-like Homeobox; OCT:
Octamer-binding Transcription Factor; AFP: Alpha-fetoprotein; PAX: Paired-Box;
DAPI: 4',6-diamidino-2-Phenylind.
INTRODUCTION
Micro RNAs (miRNA) are short (18~25 nucleotides) non-coding RNAs
generated from genomic sequences. MiRNAs mainly work as negative
post-transcriptional regulators by binding to 3’UTRs of target mRNAs, even on
condition of imperfect matches. Hence, a single miRNA can target multiple mRNAs
and miRNAs are involved in various biological pathways [2]. Accumulating
studies have revealed that miRNAs are important molecules for modulating
pluripotency [3] and differentiation [4] of hES/hiPS cells. For example, neural
stem cell proliferation and neural differentiation are regulated by expression
of miR-9, an abundant miRNA in brain [5].
MiR-27b, a somatic enriched miRNA, is one of the paralogs of miR-27,
miR-27a and miR-27b, with only one nucleotide difference. Both paralogs are
produced as polycystronic clusters of miR-23~27~24. MiR-27 functions in various
biological events such as adipogenesis, lipid metabolism [6,7] and suppression
of tumor progression [8]. As for the earlier differentiation, recent studies
have shown that increasing miR-27b expression was detected during hepatic
differentiation from hES cells [9]. Double knock-out of miR-23a/b~27a/b~24
clusters in mouse ES cells suppressed differentiation into mesodermal lineage
[10]. Other studies have revealed that over-expression of miR-27a in human
embryonal carcinoma cells negatively regulated expression of genes associated
to self-renewal and pluripotency [11]. However, the role of miR-27b in
maintenance of undifferentiated state and the differentiation into endodermal
lineage of hiPS cells has not been addressed directly.
In this study, we have generated hiPS cells, in which Doxycycline
(DOX)-inducible miR-27b-expression system was integrated by CRISPR/Cas9
technology-mediated knock-in method into AAVS1 locus. Using the hiPS cell line
combined with the in vitro hepatic
differentiation system, we report here the negative roles of miR-27b on
undifferentiation state of hiPS cells and early differentiation toward the
hepatic lineage.
MATERIALS AND
METHODS
iPS cell culture
Human iPS cell lines (Tic, JCRB Cell Bank) were cultured on mitomycin C
treated mouse embryonal fibroblast (MEF, Millipore) with ReproStem (ReproCELL),
10ng/μl fibroblast growth factor-2 (FGF-2, KATAYAMA CHEMICAL INDUSTRIES).
Hepatic differentiation
In vitro hepatic differentiation was
performed as previously described [12] with some modifications. Briefly, prior
to hepatic differentiation, hiPS cells were dissociated into single cells by
Accutase (Sigma Aldrich) and plated onto BD Matrigel Matrix Basement Membrance
Growth Factor Reduced-coated plates (Becton, Dickinson and Company) by 4.0ⅹ105
cells/ml and cultured with ReproStem, 10ng/μl FGF-2 and 10μM Rock
inhibitor (Y-27632, Sigma). The definitive endoderm cells were induced by
L-Wnt3A-expressing cell (CRL2647;ATCC)-conditioned RPMI-B27 media (RPMI 1640
media (Sigma) containing 1ⅹB27 Supplement Minus Vitamin
A(Invitrogen), 4mM GlutaMAX (Invitrogen)) with 100ng/ml Activin A (R&D
Systems) for 4 days. The formation of hepatoblasts was driven by RPMI-B27 media
containing 20ng/ml each of bone morphogenetic protein 4 (BMP4, R&D Systems)
and FGF-4 (R&D Systems) for 5 days. For heptic differentiation, the
hepatoblast-like cells were cultured with RPMI-B27 media supplemented with 20ng
of hepatocyte growth factor (HGF, R&D Systems) for 5 days, then cultured
with hepatic maturation medium (hepatic maturation medium consists of
Hepatocyte Culture Medium (HCM; Lonza, without epidermal growth factor (EGF))
containing 20 ng/mL oncostatin M (OsM) and 3% Gluta MAX) for 11 days.
Inducible gene
expression plasmid
Construction of the targeting vector is described in Figure 2A. The targeting vector was
constructed based on AAVS1 donor plasmid [13]. First, miR-27b expressing
plasmid was generated by insertion of double-stranded oligonucleotides
encompassing miR-27b into pcDNATM6.2-GW/miR plasmid (Invitrogen)
according to the manufacture’s instruction. The sequence of miR-27b was
described below. Then, restriction fragments of miR-27b cassette with upstream
emerald green fluorescent protein (GFP) were inserted into downstream of
tetracycline response element (TRE) of pTetOneTM plasmid (Clontech).
Resulting fragments of TRE-EGFP-miR-27b cassette were cloned into upstream of
elongation factor 1 alpha (EF1α) promoter in AAVS1 donor plasmid in which
enhanced green fluorescent protein (EGFP) was replaced by reverse
tet-controlled transcriptional activator (rtTA) from pTetOneTM plasmid.
Human miR-27b Top :
5’-ACCTCTCTAACAAGGTGCAGAGCTTAGC
TGATTGGTGAACAGTGATTGGTTTCCGCTTTGTTCACAGTGGCTAAGTTCTGCACCTGAAGAGAAGGTG-3’
Human miR-27b Bottom :
5’-CACCTTCTCTTCAGGTGCAGAACTTAGCCA
CTGTGAACAAAGCGGAAACCAATCACTGTTCACCAATCAGCTAAGCTCTGCACCTTGTTAGAGAGGT-3’
Generation of
Knock-In hiPS cells
The protocol for electroporation was described in the previous report
[13]. Briefly, hiPS cells were treated with 10μM valproic acid for 24 hours
before electroporation. Then, hiPS cells were harvested as single cells using
Accutase (Sigma Aldrich). Targeting plasmid (5μg), px330-AAVS1 gRNA/Cas9
expressing plasmid (5μg, construction described in previous report [13]) and
RAD51 expression plasmid (1μg, construction is described in previous report
[13]) were co-electroporated into hiPS cells (2x106) using NEPA21
electroporator (Nepagene) according to the manufacturer’s instructions. Cells
were seeded on iMatrix-511 (Nippi) coated plates in the presence of
StemFit®AK02N (ReproCELL) and 10μM Rock inhibitor. Fourty-eight hours after
electroporation, 10μg/ml puromycin was added for 48 hours to select knocked-in
hiPS colonies. Isolated colonies were picked up and expanded for preparation of
genomic DNAs. Genomic DNA was subjected to PCR to amplify the targeted genomic
regions. PCR reaction was performed using Verti thermal cycler (Applied
Biosystems). The primer sequences for PCR are depicted in Table 1. The obtained clone was designated as hiPS-AAVS1-27b. For
induction of miR-27b expression, cells were cultured in the presence of 1μg/ml
DOX.
RNA isolation and
quantative RT-PCR (qRT-PCR)
Total RNA was isolated using ISOGEN (NIPPON GENE) according to the
manufacturer’s instruction. 500ng of total RNA was used to synthesize cDNA with
a Superscript VILO cDNA synthesis kit (Thermo Fisher Scientific). qRT-PCR was
carried out with StepOnePlus real-time PCR system (Applied Biosystems) using
Fast SYPR Green Master Mix (Applied Biosystems). Results were analyzed with ⊿⊿Ct method normalized by internal reference,
GAPDH. Student t-test was performed. The primer sequences are described in Table 2.
miRNA TaqMan assay
Taqman® MicroRNA Assay Kits (Applied Biosystems)
was used for quantification of miR-27b expression according to the
manufacturer’s instruction. Briefly, 10ng of total RNA were used to perform
reverse-transcription (RT) and 1.33μl of RT products out of 7.5μl total
reaction mixture was used to qRT-PCR. Results were analyzed with ⊿⊿Ct method normalized by internal control, RNU48
(Applied Biosystems).
STATISTICAL ANALYSIS
Statistical analysis was performed with unpaired two-tailed Student’s
t-test.
Immunofluorescence
staining
The cells were fixed with 4% paraformaldehyde
(PFA, Wako) in PBS for 30 min at room temperature. After blocking and
permealising cells with PBS containing 0.2% Triton X-100 (Sigma Aldrich) and 2%
bovine serum albumin (BSA) for 45 min at 4 °C, the cells were incubated
with a primary antibody at 4 °C overnight, and finally, incubated with a
secondary antibody at room temperature for 1 hour. All the antibodies are
listed in Table 3.
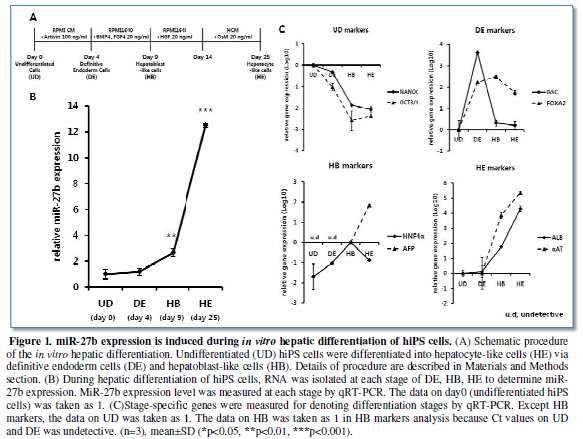
RESULT
miR-27b expression
is induced during in vitro hepatic
differentiation of hiPS cells
The previous studies have reported that miR-27b was upregulated during
hepatic differentiation from ES/iPS cells, suggesting the roles of miR-27b in
these stages [9,11]. Therefore, we examined the expression profile of miR-27b
through the differentiation according to our procedure (Figure 1A), since the time course and the definition of
differentiation stages might vary depending on protocols. MiR-27b expression
was moderately upregulated at day 4 of culture, which is the definitive
endodermal (DE) stage as denoted by the marker genes expression (Figure 1B, 1C). Afterward, significant
increase in miR-27b expression from definitive endoderm (DE) state to
hepatoblast-like cells (HB) and drastic increase to hepatocyte-like cells (HE)
were observed, confirming the previous observations with unexpectedly low
miR-27b expression during endodermal differentiation.
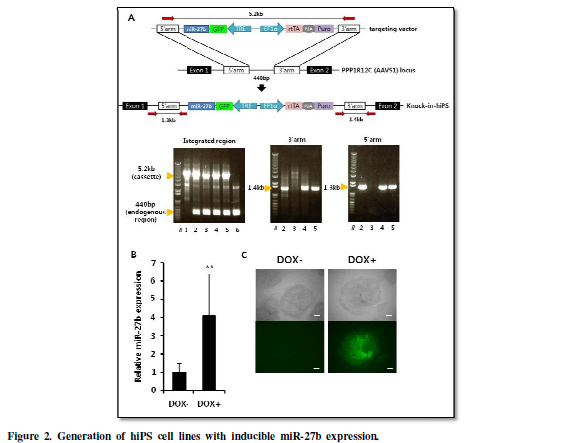
Generation of hiPS
cell lines with inducible miR-27b expression.
In order to examine whether miR-27b regulates the undifferentiated
state of hiPS cells and its differentiation towards hepatocyte, we generated
hiPS cells carrying DOX-inducible miR-27b expression system to achieve the
programmed induction of miR-27b in undifferentiated hiPS cells or during
endodermal differentiation. In order to assure stable transgene expression,
miR-27b inducible cassette was integrated into AAVS1 loci, known as
‘safe-harbor’, suitable for constitutive strong transgene expression [14], by
using CRISPR/Cas9 [15,16]. Isolated hiPS-clones were subjected to diagnostic
PCR (Figure 2A), and heterozugously
knocked-in clones, designated as hiPS-AAVS1-27b, were successfully obtained.
One of the knocked-in clones was further analyzed to confirm inducible
expression of miR-27b and its upstream GFP by treatment with DOX (Figure 2B, 2C).
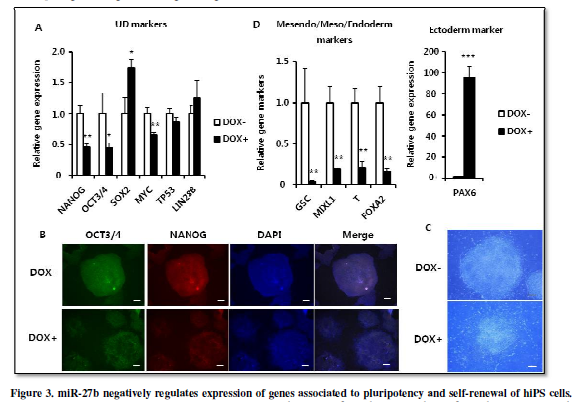
miR-27b negatively
regulates the expression of genes associated to pluripotency and self-renewal
of hiPS cells.
Previous studies have shown that miR-27a over-expression inhibits
expression of pluripotency-associated genes including OCT3/4 and LIN28B in
human embryonal carcinoma cells [11] and Oct3/4
and Nanog in mouse ES cells [10].
Therefore, we examined whether miR-27b over-expression also suppressed
expression of those genes in hiPS cells. When culturing the hiPS-AAVS1-27bwith
or without DOX for 4 days, mRNA expression of NANOG and OCT3/4 was
significantly suppressed (Figure 3A).
Immunofluorescent staining also indicated decrease of NANOG and OCT3/4 expression (Figure 3B). Furthermore, the morphological change with ambiguous edges also indicated that hiPS cells started differentiation (Figure 3C). Moreover, expression of PAX6, a gene representing ectoderm differentiation, was induced, while expression of genes associated to mesendoderm (GSC,MIXL1)/mesoderm (T)/endoderm (FOXA2) differentiation was strongly suppressed (Figure 3D). In addition, expression of SOX2 gene, known as not only for pluripotency factor but also as specific modulator to induce neural differentiation [17] was also increased (Figure 3A). Since the neuroectodermal fate is considered to be the default direction of ES/iPS differentiation, these results are well-consistent to the notion that miR-27b acts negatively to maintain the undifferentiated state of hiPS cells.
Excessive miR-27b
expression impaired definitive endodermal and mesodermal differentiation.
In the previous report, it has been shown that miR-27b expression is
upregulated during hepatic differentiation and overexpression of miR-27a in hEC
cells leads to upregulated expression of differentiation-related genes [11],
which suggested that miR-27b might also positively regulate the directed
differentiation of hiPS cells to the hepatic lineage. During the DE
differentiation, expression of miR-27b was kept relatively low, but
significantly increased afterward (Figure
1B). DOX successfully induced more than 2-fold higher expression of miR-27b
compared to the endogenous expression during the DE differentiation. However,
additive increase of miR-27b expression from the integrated cassette was not
detectable after day9 (Figure 4A). It
was probably because of silencing in AAVS1 locus during hepatic differentiation
as recently reported [18]. Therefore, we decided to examine whether higher
expression of miR-27b has some stimulatory or inhibitory effects on the DE
differentiation and eventually on hepatic differentiation. When miR-27b was
induced for the entire period of the DE differentiation (day0-4),
undiffrentiation marker expression (OCT3/4)
was not suppressed in contrast to the result in undifferentiated hiPS
cell-culture (Figure 3A, 4B),
suggesting that activin-directed initiation of differentiation was inhibited to
some extent. Importantly, mesendoderm (GSC)
and endoderm (FOXA2) markers were
significantly increased, while mesoderm marker (brachyury (T)) was strongly suppressed. These results suggested that increased
expression of miR-27b promotes the DE differentiation by suppressing mesoderm
differentiation.
Next, we divided the miR-27b induction period into two (day0-2 and
day2-4) and examined which period represents the effects of miR-27b induction
for day0-4 on the DE and mesoderm differentiation. When miR-27b was induced for
the earlier period (day0-2), the mesoderm marker (T) was suppressed, while mesendoderm marker (GSC) was increased similarly to day0-4. On the other hand, when
miR-27b was induced for the later period (day2-4), the DE-specific marker (FOXA2) was suppressed, while mesendoderm
(GSC, MIXL1) and mesoderm (T)
markers were increased (Figure 4B).
These results revealed that miR-27b induction during endoderm formation,
particularly during earlier period of formation, contributes positively to the
DE and negatively to mesoderm differentiation, and that the later induction
caused the opposite effects.
Excessive miR-27b
expression during endodermal differentiation suppressed hepatoblast and hepatic
differentiation.
MiR-27b induction during earlier endodermal differentiation caused
somewhat increasing of endoderm marker expression and significant suppression
of mesoderm marker expression, while later induction caused increase in
mesodermal marker (Figure 4B). We
next examined the influences of these changes in the DE and mesodermal
differentiation on later developmental stages. Both hepatoblast (HNF4α, AFP) and hepatocyte (ALB)
gene expression were suppressed by miR-27b induction for day2-4 (Figure 5A, 5B), indicating that
suppression of the DE differentiation and stimulation of mesodermal
differentiation at day4 lead to the inefficient hepatic differentiation. In case
of miR-27b induction for day0-4 and day0-2, we predicted stimulation of hepatic
differentiation because expression of DE markers was upregulated. However,
hepatic differentiation was clearly suppressed in both cases as marker gene
expression (αAT, ALB) was decreased (Figure
5B). Taken altogether, these results suggested that properly restricted
expression of miR-27b during endoderm differentiation was crucial for in vitro hepatic differentiation of hiPS
cells.
DISCUSSION
In this report, we generated DOX-inducible miR-27b-expressing hiPS cell
lines, and uncovered the role of miR-27b for maintaining pluripotency and for
proper regulation of the endodermal differentiation in hiPS cells. We found
that induced miR-27b expression caused strong suppression of pluripotency-associated
gene expression in hiPS cells with morphological changes of hiPS colonies (Figure 3A, 3C). In addition, genes
associated to ectoderm, the default direction of iPS differentiation, were
significantly increased, while mesendoderm/mesoderm/endoderm marker expression
was suppressed (Figure 3D). These
results supported the previous studies, in which overexpression of miR-27b in
hEC cells suppressed undifferentiation-related markers, even more convincingly
as we employed hiPS [11]. Also, deletion of miR-24a/b~27a/b~24 clusters in
mouse ES cells was reported to have no obvious effects on gene expression and
colony formation but impaired differentiation of embryoid bodies [10]. Thus,
our studies along with others’ established the role of miR-27b as a negative
regulator against the undifferentiated state of cells and, therefore, miR-27b
should be suppressed to maintain ES/iPS cells in undifferentiated state.
MiR-27 has been reported to repress expression of SMAD2/3, a downstream
component of activin [11]. As activin was used as a critical cytokine to direct
endodermal differentiation in in vitro
hepatic differentiation, the impaired differentiation might be explained by
inhibitory effect of miR-27b on NODAL signal. MiR-27b induction at the beginning
of differentiation might inhibit NODAL signal required for the initiation of
differentiation program leading to increase in undifferentiation
marker-expression (Figure 4B).
Similarly, induced expression of miR-27b in later period (day 2-4) might weaken
the NODAL signal. As the high level of NODAL signal is the inducer of endoderm
differentiation and the low level stimulates mesoderm differentiation [19,20],
the NODAL signal, weakened by miR-27b induction, might stimulate mesoderm
differentiation but suppress the DE differentiation as we observed (Figure 4B).
We found that miR-27b expression was increasing
during hepatic differentiation, which was consistent to the previous findings
[9,11], but unexpectedly, stayed relatively low level in formation of DE (Figure 1B). We also found that the
induced miR-27b expression during endoderm differentiation, regardless the
inducing periods, eventually caused suppression of hepatoblast- and
hepatic-differentiation (Figure 5A).
In total, these findings uncovered the importance of secured low expression of
miR-27b for both endodermal and mesodermal differentiation and consequently
formation of hepatoblast-like cells, the progenitor of hepatic differentiation,
and hepatocyte-like cells, which further confirms the importance of suppression
of miR-27b expression in endodermal differentiation to ensure the hepatocyte
development.
CONCLUSION
In summary, we revealed the role of miR-27b involving in endodermal
differentiation as well as in maintenance of undifferentiation state in hiPS
cells. Suppression of miR-27b in early stages is required to keep pluripotency
and self-renewal, and to secure correct differentiation of hepatocytes via
endoderm formation. The specific role of miR-27b in late differentiation stages
still remains to be elucidated. Additional study is necessary to identify how
miR-27b regulates hepatic differentiation and clarification of its molecular
pathway would contribute to understand underlying mechanism of embryonic
development.
ACKNOWLEDGMENTS
We thank Dr. Akitsu Hotta (Center for iPS Cell Research and
Application, Kyoto University) for providing pENTR donor plasmid to design
AAVS1 donor plasmid, Ms. Y. Hagihara (Graduate School of Pharmaceutical
Sciences, Osaka University) for technical supports and Mr. Marcos Taracena
(Graduate School of Pharmaceutical Sciences, Osaka University) for critical
reading of the manuscript. This study was supported by Research Program on
Hepatitis from Japanese Agency for Medical Research and Development (AMED)
Japan.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
J.L. performed the experiments, analyzed data,
and wrote the manuscript. E.S. designed the experiments and wrote the
manuscript. K.T. assisted to design and perform the experiments. F.S.
supervised the project. H.M. supervised the project and wrote the manuscript.
- Si-Tayeb
K, Noto FK, Nagaoka M, Li J, Battle MA, et al. (2010) Highly efficient
generation of human hepatocyte-like cells from induced pluripotent stem
cells Hepatology. 51 (2010) 297–305.
- Bartel
DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell
116: 281-297.
- Mallannaa SK, Rizzino A (2010) Emerging roles of microRNAs in the
control of embryonic stem cells and the generation of induced pluripotent
stem cells. Developmental Biology 334: 16-25.
- Yao
S (2016) MicroRNA biogenesis and their functions in regulating stem cell
potency and differentiation. Biol Proced Online 18. 8. s12575-016-0037-y.
- Zhao
C, Sun G, Li S, Shi Y (2009) A feedback regulatory loop involving
microRNA-9 and nuclear receptor TLX in neural stem cell fate
determination. Nat Struct Mol Biol 16: 365-371.
- Michael
K, Fischer C, Nowitsch S, Opriessnig P, Papak C, et al. (2009) microRNA
miR-27b impairs human adipocyte differentiation and targets PPARγ. Biochem Biophys Res Commun
390: 247-251.
- Kasey
VC, Shoucri BM, Levin MG, Wu H, Pearson DS, et al. (2013) MicroRNA-27b is
a regulatory hub in lipid metabolism and is altered in dyslipidemia.
Hepatology 57: 533-542.
- Lee
JJ , Drakaki A, Iliopoulos D, Struhl K (2011) MiR-27b targets PPARγ to inhibit growth, tumor progression
and the inflammatory response in neuroblastoma cells. Oncogene 31:
3818-3825.
- Kim
N, Kim H, Jung I, Kim Y, Kim D, et al. (2011) Expression profiles of
miRNAs in human embryonic stem cells during hepatocyte differentiation.
Hepatol Res 41: 170-183.
- Ma
Y, Yao N, Liu G, Dong L, Liu Y, et al. (2015) Functional screen reveals
essential roles of miR-27a/24 in differentiation of embryonic stem cells.
EMBO J 34: 361-378.
- Fuchs
H, Theuser M, Wruck W, Adjaye J (2014) MIR-27 Negatively Regulates
Pluripotency-Associated Genes in Human Embryonal Carcinoma Cells. PLoS One
9:
e111627.
- Takayama
K, Morisaki Y, Kuno S, Nagamoto Y, Harada K, et al. (2014) Prediction of
interindividual differences in hepatic functions and drug sensitivity by
using human iPS-derived hepatocytes. Proc Natl Acad Sci USA 111:
16772-16777.
- Takayama
K, Igai K, Hagihara Y, Hashimoto R, Hanawa M, et al. (2017) Highly
efficient biallelic genome editing of human ES/iPS cells using a
CRISPR/Cas9 or TALEN system. Nucleic Acids Res: 1-10. doi:10.1093/nar/gkx130.
- Smith
JR, Maguire S, Davis LA, Alexander M, Yang F, et al. (2008) Robust,
Persistent Transgene Expression in Human Embryonic Stem Cells Is Achieved
with AAVS1-Targeted Integration. Stem Cells 26: 496-504.
- Hsu
PD, Lander ES, Zhang F (2014) Development and applications of CRISPR-Cas9
for genome engineering. Cell 157: 1262-1278.
- Cong
L, Ran FA, Cox D, Lin S, Barretto R, et al. (2013) Multiplex Genome
Engineering Using CRISPR/Cas Systems. Science 339: 819-23.
- Zhang
S, Cui W (2014) Sox2, a key factor in the regulation of pluripotency and
neural differentiation. World J Stem Cells 6: 305-311.
- Ordovás L,
Boon R, Pistoni M, Chen Y, Wolfs E, et al. (2015) Efficient
recombinase-mediated cassette exchange in hPSCs to study the hepatocyte
lineage reveals AAVS1 locus-mediated transgene inhibition. Stem Cell
Reports 5: 918–931.
- Takenaga
M, Fukumoto M, Hori Y (2007) Regulated Nodal signaling promotes
differentiation of the definitive endoderm and mesoderm from ES cells. J
Cell Sci 120: 2078-2090.
- S.D. Vincent,
Dunn NR, Hayashi S, Norris DP, Robertson EJ (2003) Cell fate decisions
within the mouse organizer are governed by graded Nodal signals. Genes Dev
17: 1646–1662.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Alcoholism Clinical Research
- Ophthalmology Clinics and Research (ISSN:2638-115X)
- International Journal of AIDS (ISSN: 2644-3023)
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)