1215
Views & Citations215
Likes & Shares
OBJECTIVES
- Propose a fair cost for Ivonescimab and compare with ICI and TT.
- Heed the warning of the high costs of prolonged TT use.
- Chart a pathway to ensure drug affordability and cost-saving.
DRUGS AND METHODS
The annual 2022-2024 drug costs were abstracted and calculated in United States (US) dollars. All the analyzed drugs are currently approved and used in Europe, Canada and Europe.
RESULTS
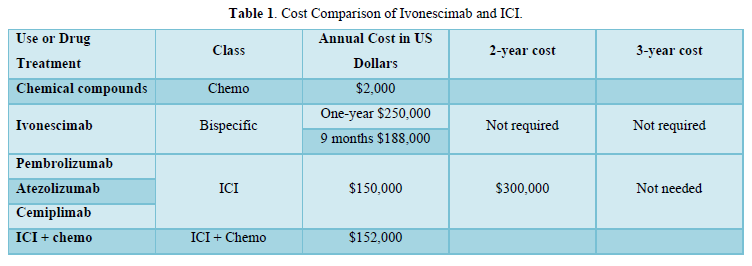
An annual $250,000 Ivonescimab cost and 9-month of $188,000 were proposed in view of the reported value in a/m-NSCLC, originality and sophisticated multistep synthesis (HARMONi-2). The ICI were introduced approximately 10 years ago, with Pembro being the 1st reported in treatment of a/m-NSCLC with programmed cell death protein 1 (PD-1) > 50%. The estimated average cost per patient per year was $150,000. Nivolumab, Durvalumab and Cemiplimab soon followed at essentially comparable
costs. The ICI 2-year $300,000 cost was equitable with the documented overall survival. The 3-year use was unwarranted due to lack of further survival improvement. The addition of low-cost chemo has obviated the 50% PD-1 requirement at suggested $2,000 cost. Appropriate biomarker testing in lung cancer management is essential as prerequisite prior any ICI or TT treatment. Prior chemotherapy is sometimes utilized. The incidence of oncogenic driver mutations varies in NSCLC. It takes 2-4 tests for identification throughout the entire disease course at $4000-$8000 cost. Costs of the TT class are relatively high, and continued use is financially burdensome. The case of 65 yo female was previously reported in 2014 with a/d-NSCLC and anaplastic lymphoma kinase (ALK+) with liver, lung and bone metastasis [10]. She was successfully treated by the 2nd generation Alectinib 600 mg po bid daily with essential clearing of liver and lung involvement. Alectinib cost in 2013-14 was unaffordable at $220,000, even with insurance coverage, putting a financial pressure on the whole family. In 2023-2024, the price dropped after Alectinib losing patency to $20,000. In general, patent TT is costly because of the limited number of eligible patients and low incidence of the appropriate oncogenic drivers involved. The median cost of Osimertinib, Entrectinib, Larotrectinib and Lorlatinib and Adagrasib was $211,000. The high costs of TT continued use are illustrated in Tables 1 & 2.
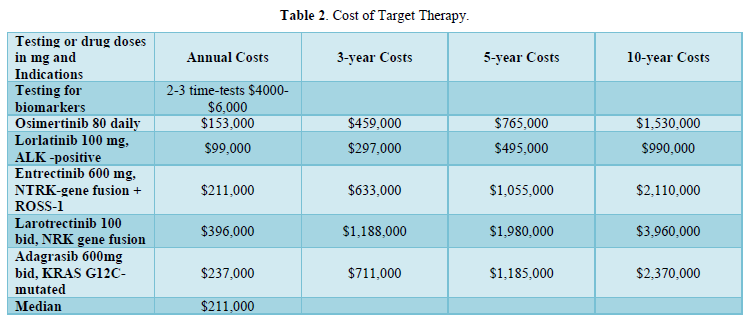
- Osimertinib [11,12] is widely used as neoadjuvant, adjuvant and in a/d NSCLC at $153,000.
- The third-generation ALK inhibitor Lorlatinib demonstrated an impressive' 5-year progression-free survival in ALK+ NSCLC (CROWN) trial as compared with crizotinib. It was also associated with a 92% reduction in intracranial progression [13]. The average annual Lorlatinib cost was justified at $99,000. c-Entrectinib is an inhibitor of the tropomyosin receptor kinases (TRK).
- ros oncogene 1 (ROS1) and anaplastic lymphoma kinase (ALK) fusion and the chimeric tropomyosin receptor kinases (TRK) fusion. Estimated annual cost $210,000.
- Larotrectinib inhibits the TRK fusion at an incidence of <1% and annual $396,000 cost.
- Approximately 15% of patients with non-squamous NSCLC have an actionable KRAS G12C mutation. Adagrasib, effective against such mutation. Costs $237,000 per year seem worth it. Deucravacitinibm, tyrosine kinase inhibitor (TKI), included for cost comparison, is widely used for the treatment of moderate to severe plaque psoriasis at annual $14,400 cost.
DISCUSSION
Drug costs are widely variable and aggressively negotiated. Value is established on the drug 1st approval There appears no valid reason why Ivonescimab value could not be universally confirmed. In PD-1 > 1%, the progression-free survival was significantly longer than Pembro at similar safety profiles. The study was analyzed at 8.7 months with impressive 0.54 hazard ratio. Many years of follow-up are still needed with more questions in search of answers. What happens after 8.7 months? How and when do the 2 curves look or meet? Is there an overall survival? Regardless, the proposed 9-months cost of $188,000 is worthy it. However, Ivonescimab should not be used longer than 9 months. The ICI have been widely used as neo-adjuvant, adjuvant, consolidation and in the treatment of NSCLC at an annual $150,000 cost. Pembro has been on the market for about 10 years and would later lose patency with another ICI to follow. The drug company is planning to produce a subcutaneous formulation instead of intravenous injections. Nivolumab + Ipilimumab combination is widely used and Ipilimumab + Relatlimab is being explored. In general, the lower the incidence of oncogenic driver, the lower is the number of eligible patients for TT use. The longer the use beyond the indicated 2-3 years, the higher is the cost. At 5-years, Osimertinib [11,12] was considered valuable for the overall survival reported. It is advisable, however, for economic reasons, to restrict Osimertinib use beyond 5-years till proof of further survival established. Cost of drugs, milk or bread was previously reported to be determined by the total number of purchases, and not limited by the 1st transaction [9]. The saving on the first purchase would multiply with further transactions. Many attempts to halt or at least mange the rising drug costs have failed. Caps utilization has been poorly received [14].
Value of other TT 2-3-year were proven and reported during the approval criteria. Beyond 2-3 years, costs rapidly multiply. In case of recurrence, the drug could be re-introduced and tested. In case of failure, another class as antibody-drug conjugate or bispecific be tried. Clinical studies are needed to establish activity and safety. At present, Ivonescimab is a pioneer and hopefully more drugs of the bispecific class are yet to come. The role of the medical insurance companies has to be carefully investigated. Refusal of payments and/or partial payments need to be scrutinized.
SUMMARY
The development of the bispecific class in general, and Ivonescimab in particular is a paradigm shift in the treatment of a/d-NSCLC cancer. The suggested 9-month $188,000 cost was fair and equitable with its reported value. Osimertinib 5-year use cost is consistent with its proven overall survival. The 10-year use is not warranted at present due to lack of further survival. Other TT 2-3-year ought to be strictly used within their approval terms. Extended use presents a direct threat to the financial liquidity of the national and international health economy. Affordability benefits both manufacturers and consumers by promoting further use. Drug costs need be aggressively negotiated. Pharma has created values drugs. The growing financial toxicity of prolonged therapy has been previously described (15,16). Oncologists bear most of the financial burden by using anticancer drugs longer and outside their indications. A pathway has been deployed to ensure drug affordability and cost-saving.
CONCLUSION
The Ivonescimab 9-months cost of $188,000 is fair and equitable to its value. Extended TT is too burdensome to bear and ought to be avoided. A pathway for drug affordability and cost saving was chartered as a global vision.
- Zhou C, Chen J, Wu L, Chen J, Xia M, et al. (2024) Phase 3 study of ivonescimab (AK112) vs pembrolizumab as first-line treatment for PD-L1-positive advanced NSCLC: Primary analysis of HARMONi-2. 2024 World Conference on Lung Cancer.
- Wang L, Luo Y, Ren S, Zhang Z, Xiong A, et al. (2024) A phase 1b study of ivonescimab, a programmed cell death protein-1 and vascular endothelial growth factor bispecific antibody, as first- or second-line therapy for advanced or metastatic immune-therapy-naive NSCLC. J Thorac Oncol 19: 465-475.
- Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blum D, et al. (2015) American Society of Clinical Oncology statement: A framework to assess the value of cancer treatment options. J Clin Oncol 33(23): 2563-2577.
- Cherny NI, Sullivan R, Dafni U, Kerst J, Sobrero A, et al. (2015) A standardized, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol 26(8): 1547-1573.
- Rome BN, Kesselheim AS, Feldman WB (2024) Medicare’s First Round of Drug-Price Negotiation-Measuring Success. N Engl J Med 391(20): 1865-1868.
- Garon EB, Rizvi NA, Hui R, Leighl N, Aggarwal C, et al. (2015) Pembrolizumab for the treatment of non-small cell lung cancer. N Engl J Med 372(21): 2018-2028.
- Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, et al. (2016) Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375(19): 823-1833.
- Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, et al. (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-postive, advanced non-small-cell lung cancer (KEYNOTE -010): A randomized controlled trial. Lancet 387: 1540-1550.
- Guirgis HM (2024) Costs of Target Therapy and Proportionality to Number of Purchases: Propose Using Maintenance Dose and Limited Duration. Med Res Arch 12(1): 1-5.
- Guirgis HM (2023) Cost matters. J Cancer Oncol 7(1).
- Herbst RS, Wu Yi-long, John T (2023) Adjuvant Osimertinib for Resected EGFR-Mutated Stage IB-IIIA Non–Small-Cell Lung Cancer: Updated Results from the Phase III Randomized ADAURA Trial. J Clin Oncol 41(10): 1830-1840.
- Lv C, Fang W, Wu N, Jiao W, Xu S, et al. (2023) Osimertinib as Neoadjuvant Therapy for EGFR-Mutant Resectable Stage II-IIIB Lung Adenocarcinoma. Lung Cancer 178: 151-156.
- Solomon B, Liu G, Felip E, Bauer TM, Shaw AT, et al. (2024) Lorlatinib Versus Crizotinib in Patients with Advanced ALK-Positive Non-Small Cell Lung Cancer: 5-Year Outcomes from the Phase III CROWN Study J Clin Oncol 42(29): 3400-3409.
- Guirgis HM (2023) Target Therapy vs the Immune Check Point Inhibitors in Guirgis, HM. Target Therapy vs the Immune Check Point Inhibitors in Lung Cancer, costs and caps platform.
- Li M, Liao K, Pan I-Wen, Tina Shih YC (2002) Growing Financial burden from high cost targeted oral anticancer medicines among Medicare beneficiaries with cancer. JCO Oncol Pract 18(11): e1739-e1749.
- Peppercorn J (2023) The impact of financial toxicity on cancer care. Clin Adv Hematol Oncol 21(10): 520-523.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Diabetes (ISSN: 2644-3031)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)


