1116
Views & Citations116
Likes & Shares
Human obesity differs from that of rodents and animals
which are usually utilized for studying both aetioathogenesis along with
treatment of obesity. This is in view of hypothalamus generally considered to
be the organ for homeostatic control is under control by various supra
homeostatic control besides peripheral regulation. Hence the needs to
understand how the higher centres regulate hyothalamus for a given response.
Various functional neuroimaging studies have proved to be helpful in depicting
these roles. Further it has helped in understanding how the most effective
treatment like bariatric surgery (BS) alters these supra hypothalamic control
in altering hippocampus, areas involved in executive function along with
reducing ghrelin besides changing the cortical
thickness with alterations of ratios of gray and white matter. These changes
need to be translate to drugs utilized for anti-obesity therapy as has been
done by lorcaserin, liraglutude and other natural food products like walnuts
and other such natural products to help better get some safe options for
anti-obesity therapies that prove to be clinically effective.
Keywords:
Hypothalamus, Learning and memory, Hippocampus, Attention systems, Cognitive
control, BS, Anti-obesity drugs
INTRODUCTION
Obesity has become a worldwide health
epidemic as declared by World Health Organization (WHO) in 2003 especially in
industrialized countries, where, >1/3rd population is obese and
1/3rd people are overweight [1]. Since, eating in humans not only
controlled by hypothalamus, we tried to study the role of higher CNS centres
involved in eating, to have a better understanding of aetioathogenesis of
obesity.
MATERIAL AND METHODS
We did a
PubMed study of MeSH Terms like hypothalamus, reward and memory in obesity,
attention, cognitive control of eating, besides various neuroimaging studies
pertaining to obesity from 1990-2018.
RESULTS
We found a
total of 382 articles pertaining to this. Considering the duplicate nature of
some articles we selected 51 articles for this mini-review. No meta-analysis
was done.
Role of hypothalamus
Although most
of our concentration has been focused on studying role of hypothalamus in
obesity with the findings of orexigenic neuropeptide Y(NPY)/Agouti Related
Protein (AgRP) neurons and anorexigenic proopio melanocortin (POMC)/Cocaine and
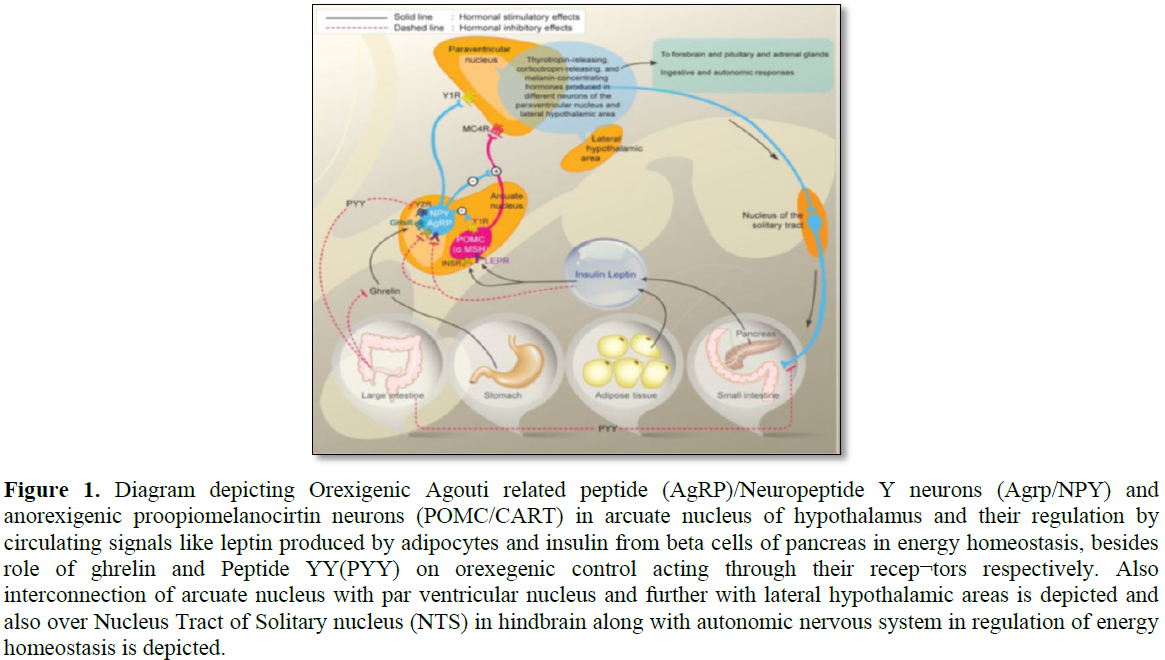
Amphetamine Related Transcript (CART) neurons in the arcuate nucleus (Figure 1) [2-4] and further orexigenic
neurons in Lateral Hypothalamus (LH), paradoxical stimulation results
in rats and
monkeys over
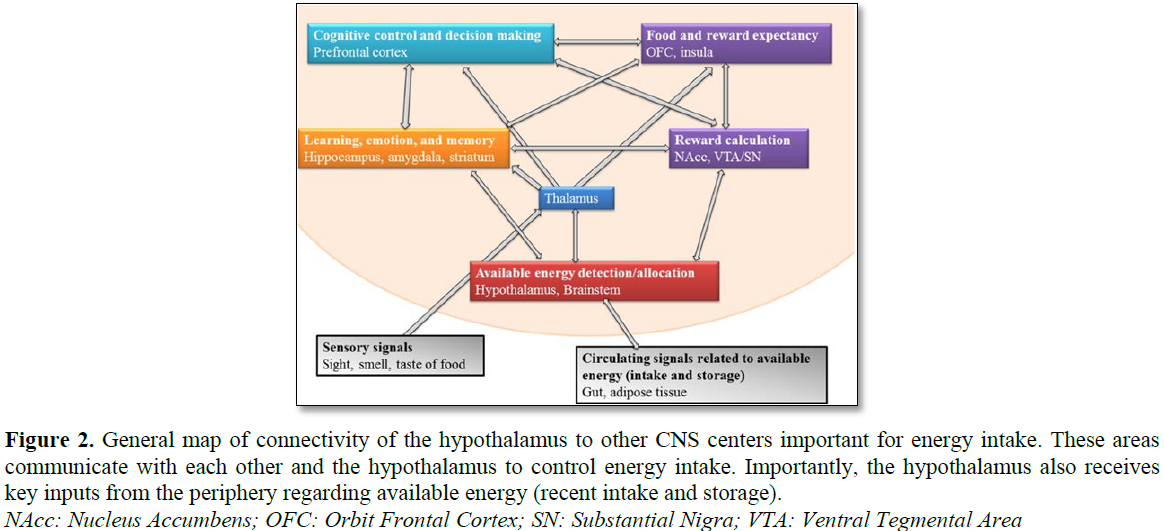
Role of reward
systems (Figure 3)
Main theories regarding how these get changed
in obesity is: i) Hyporesponsitivity to reward-as summarized by PET studies
have shown lower availability of dopamine D2 receptors in striatum in obese as
compared to normal weight rats along with same findings in humans as well. Thus
suggestion is lower dopaminergic signal might => people to seek highly
rewarding foods that in turn => obesity. An under responsive reward circuit
and habitual food intake of high fat/calorie foods has been compared to drugs
of addiction as reviewed earlier [2,6-12].
Other theory: ii) Hyper responsitivity to
food cues => individuals to seek more and more in quantity. Increased
exploration of these highly rewarding foods => larger disconnect between
reward exposure to food cues and response to consuming foods which => them
to eat more foods to achieve expected reward. This has been supported by
activation of nucleus accumbens, midbrain and Orbitofrontal Cortex (OFC) in
visual foods cues and to achieve the expected reward anticipation of milk shake
[13].
Role of emotion and
memory
The amygdala is the primary one that
regulates appetite in response to emotions. Amygdala activates to food cues
[14,15] and this response increases in childhood, adolescent and adult obesity
[16-19]. Activation of amygdala also predicts consumption of high fat or high
calorie foods [20]. Participants with responses of amygdala to food cues when
not hungry had chances of gaining weight [21]. Higher levels of leptin in
adolescents correlated with activation of amygdale to high calorie foods [19].
Stress relieving effects of sucrose gets mediated via amygdalar circuit
communicating with hypothalamo-pituitary-Adrenal (H-P-A) axis [22].
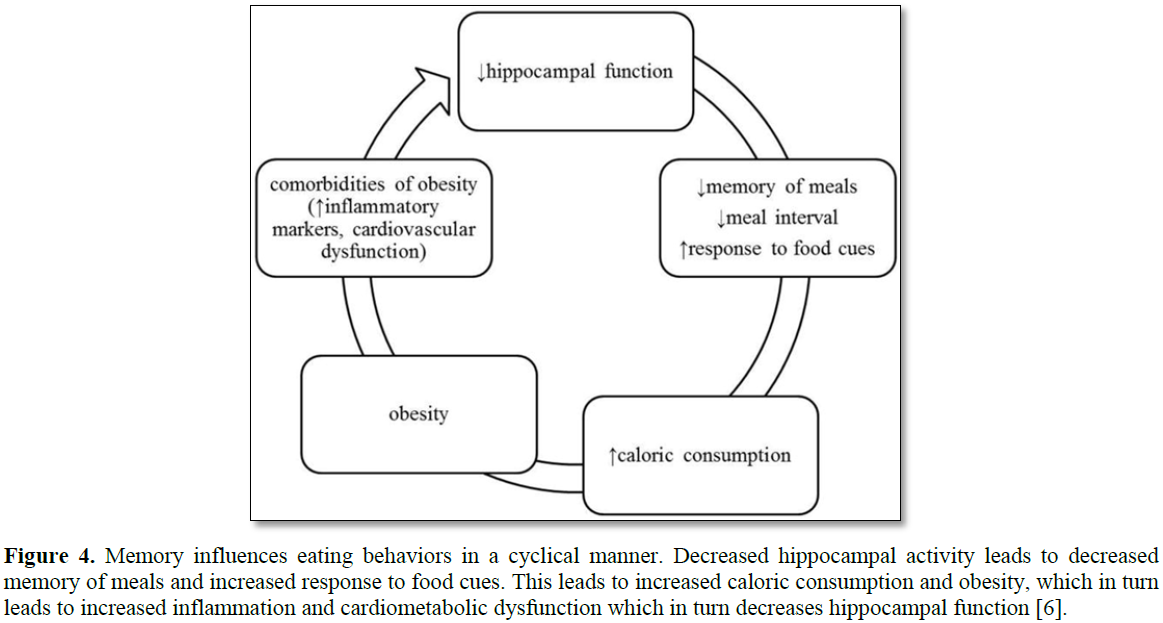
Memory is mainly regulated by hippocampus and
parahippocampal formation that might influence eating. Reduced functioning of
hippocampus => increased food intake and poor diet quality [23,24]. Though
typical timing of food gets controlled by circadian rhythms and suprachiasmatic
nucleus, evidence supports this gets over ridden by memory along with
experiences (Figure 4) [24].
Hippocampus gets inputs regarding food cues from many other areas that include
insula, OFC, as well as arcuate nucleus of hypothalamus. Further hippocampus
gets controlled by peripheral signals like leptin and ghrelin to regulate food
intake. Obesity, possibly could comprise hippocampus functions via Blood Brain
Barrier (BBB), although hippocampus is protected by BBB, cytokines associated
with inflammation may not be able to reach hippocampus, yet there is evidence
that inflammation in CNS might be carried out by microglia like in hypothalamus
[4,25,26]. In obesity, microglial action has been shown to impair function of
hippocampus are through CNS inflammatory processes. These effects on
hippocampus have been seen more in rodents than human brain with more studies
required in humans.
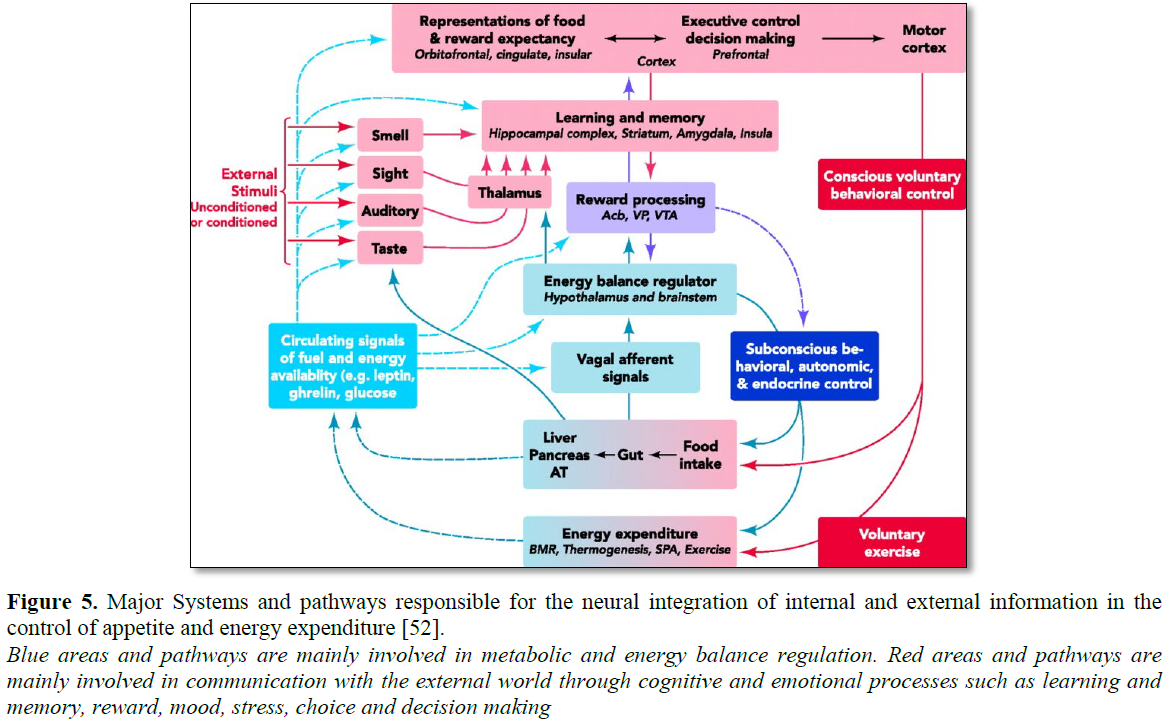
Attention systems
Brain network of attention systems include
parietal and visual cortices, along with some areas of frontal cortex [27,28].
Increased activation of occipetal cortex has been shown for high calorie/HFD
images (Figure 5).
ROLE OF COGNITIVE
CONTROL SYSTEMS
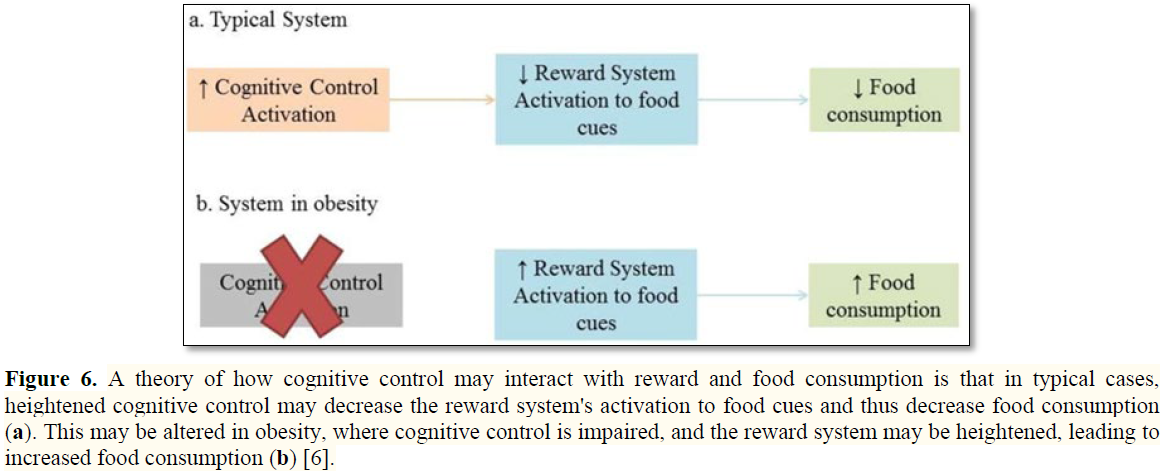
Cognitive Control Systems have executive functions including inhibition of prepotent responses. Cognitive Control allows a person to refuse a piece of cake if not feeling hungry. Prefrontal Cortex (PFC) makes most part of Cognitive Control networks especially the cingulate cortex, inferior frontal cortex, pre-supplementary motor area and dorsolateral PFC (DLPFC) [29]. Impaired inhibitory control has been shown in obese humans. A proposal has been given that impaired Cognitive Control might affect increased reward responses to food cues and thus overeating [30,31]. Less metabolism has been seen in obese as measured by Positron Emission Tomography (PET) imaging or reduced activity in PFC that correlates with dopamine receptor availability and BMI [11,32]. Thus deficits in Cognitive Control have been seen both in general and food specific tasks in obese people though it is not clear whether poor cognitive or inhibitory control is caused by obesity or causes obesity (Figure 6).
PRACTICAL
IMPLICATIONS IN THERAPEUTICS
Role of bariatric
surgery (BS)
Li et al. [33] tested the hypothesis that
Laparoscopic Sleeve Gastrectomy (LSG) induced reductions in appetite and total
ghrelin levels were associated with reduced prefrontal reactivity to food cues.
A fMRI cue related task with high calorie (HC) and low calorie (LC) food
pictures were used to determine the brain connectivity in 22 obese participants
tested before and 1 month after BS.19 other controls (Ctrl) without surgery
were also tested at baseline and 1 month later. LSG significantly decreased: i)
fasting plasma concentrations of total ghrelin, leptin and insulin ii) Craving
for HC food and iii) brain activation in the right DLPFC in response to HC vs.
LC food cues (PFWE<0.05). LSG-induced reduction in DLPFC activation to food
cues were positively correlated with reduction in ghrelin levels and reduction
in craving ratings for food. Psychophysiological interaction (PPI) connective
analysis with the central anterior cingulated cortex (vACC) after LSG and
changes in BMI were negatively correlated with changes in connectivity between
the right DLPFC and vACC in the LSG group only. Thus these findings suggest
that LSG induced weight loss may be related to reductions in ghrelin, possibly
leading to decreased food cravings and hypothetically reducing DLPFC response
in the HC food cues [33].
Zhang et al. [34] measured brain activity
with Amplitude of Low Frequency Fluctuations (ALFF), captured with resting
state fMRI in 30 obese participants both before and after 1month of LSG and in
26 obese controls without surgery that were studied at baseline and 1 month
later. A 2 way analysis was performed to model the groups and time effects on
ALFF and state functional connectivity. Significant decreases in appetite, BMI,
fasting plasma ghrelin and leptin levels, anxiety and ALFF in hippocampus
(HIPP) and ALFF increased in Posterior Cingulate Cortex (PCC, PFWE<0.05), 1
month post LSG. Decreases in HIPP ALFF correlated with decreases in anxiety and
increases in PCC ALFF correlated positively with decreases in anxiety. Seed
voxel correlation analysis showed stronger connectivity between HIPP and insula
and between PCC and DLPFC post LSG. Thus, concluding that ghrelin effects in
HIPP modulate connectivity with the insula which processes interoception and
might be relevant to LSG induced reductions in appetite/anxiety. Role of LSG in
PCC and its enhanced connectivity with DLPFC in improving self-regulation
following LSG needs further investigation [34].
Obesity related brain gray (GM) and white
matter (WM) abnormalities have been reported in regions associated with food
intake and cognitive – emotional regulation. BS is the most effective way to
treat obesity and induce structural recovery of GM/WM density and WM integrity.
Structural MRI and surface based morphometry analyses were used to investigate
BS induced alterations of cortical morphometry in 22 obese participants who
were tested before and one month post BS and in 21 obese controls (Ctrl)
without surgery who were tested twice (baseline and one month). Results showed
that fasting plasma ghrelin, insulin and leptin levels were significantly
reduced post BS (P<0.001). Post BS significant decreases in cortical
thickness in the precuneus (PFDR<0.05) that were associated with decreases
in BMI. There were also significant increases post-BS in cortical thickness in
middle (MFG) and superior frontal gyri (SFG) superior temporal gyrus (STG),
insula and vACC; and n cortical volume in left post central gyrus (Post Cen)
and vACC (PFDR<0.05). Post BS changes in SFG were associated with decrease
in BMI. These findings suggest that structural changes in brain regions
implicated in executive control and self-referential processing are associated
with BS-induced weight loss [35].
Further Li et al. [36] tried to understand
the brain alterations of Resting State Functional Connectivity (RSFC) of
Resting State Networks (RSNs) related to food intake and influence of BS on
these. They used Functional Connectivity Density (FCD) mapping to calculate
local ((FCD/Global (gFCD) voxel wise connectivity matrices in 22 obese
participants who underwent fMRI before and 1 month after sleeve gastrectomy
(SG) and in 19 obese controls (Ctrl) without surgery but tested twice (baseline
and 1 month later). Two factors (group time) repeated measures ANOVA was used to
assess main and interaction effects in IFCD/gFCD regions of interest were
identified for subsequent seed to voxel connectivity analysis to assess RSFC
and to examine association with weight loss. BS significantly reduced IFCD in
VMPFC, PCC/precuneus and dorsal Anterior Cingulate Cortex (dACC)/Dorsomedial
Prefrontal Cortex (DMPFC) and decreased gFCD in VMPFC, right dorsolateral PFC
(DLPFC) and right insula (PFWE<0.05). IFCD decreased in VMPFC, PCC and
precuneus correlated with reduction in BMI after surgery. Seed to voxel
connectivity analysis showed that the VMPFC had stronger connectivity with left
DLPFC and weaker connectivity with hippocampus/parahippocampus and
PCC/precuneus had stronger connectivity with right caudate and left DLPFC after
surgery. BS significantly decreased FCD in region as involved in
self-referential processing (VMPFC, DMPFC, dACC and precuneus) and
interoception (insula) and changes in VMPFC/precuneus were associated with
reduction in BMI suggesting a role in improving control of eating behaviors
following surgery [36].
Role of anti-obesity
drugs
Furthermore Lor caserin, a 5T 2c receptor
agonist has been found to be effective in treating obesity in humans. 5T 2c
receptors are located almost only in the CNS, which includes thalamus and
hypothalamus, areas known to be involved in feeding regulation but also in more
cortical areas involved in higher though and top down processes [37-39]. Thus
Farr et al. [40] conducted a randomized, placebo controlled blind trial with 48
obese participants using fMRI to study the effects of lorcaserin on the brain.
Subjects taking lorcaserin had decreased brain activations in the attention
related parietal and visual cortices in response to highly palatable foods cues
at one week in fasting state and in the parietal cortex, 4 weeks in response to
any cues in the fed state. Decreases in calorie intake, weight and BMI
correlated with activations of the amygdala, parietal and visual cortices at
baseline. Thus indicating that lorcaserin exerts its weight reducing effects by
decreasing attention related brain activations to food cues (parietal and visual
cortices) and emotional and limbic activity (insula, amygdala). This indicates
that baseline activation of amygdala relates to increased efficacy that
suggests that Lorcaserin would be of particular benefit in obesity associated
with emotional states [40].
Similarly Liraglutide a glucagon like peptide
1 (GLP1) analogue has been approved both for treatment of both T2DM and obesity
[2,3]. Farr et al. [41] investigated if receptors are expressed in human brains
and if Liraglutide administration affects neural responses to food cues in
diabetic individuals which were the primary outcome. Thus they studied
consecutively 22 human brains, examined expression of GLP1 receptors in the
hypothalamus, medulla oblongata and parietal cortex using immunohistochemistry.
In a randomized (assigned by pharmacy using a randomization enrollment table),
placebo controlled double blinded cross over trial, 21 individuals with T2DM
(18 included in analysis due to lack of poor quality of data) were treated with
placebo and Liraglutide for a total of 17 days each (0.6 mg for 7 days, 1.2 mg
for 7 days and 1.8 mg for 3 days). Participants were eligible if they had T2DM
and were currently on lifestyle changes or metformin for treatment.
Participants, caregivers, people doing measurement and/or examinations and
people assessing the outcomes were blinded to the medication assignment. Both
metabolic changes along with neurocognitive and neuroimaging (fMRI) of
responses to food cues were studied by them. Immunohistochemical analysis showed
the presence of GLP1 receptors on neurons in the human hypothalamus, medulla
and parietal cortex. Liraglutide decreased activation of the parietal cortex in
response to highly desirable (vs. less desirable) food images (p<0.001:
effecr size: placebo 0.53+-0.24, luraglutide-0.47+-0.18). No significant side
effects were noted. In a secondary analysis, they found reduced activation of
insula and putamen, areas involved in the reward system. Further, they showed
increased ratings of hunger and appetite correlated with increased brain
activation in response to highly desirable food cues while on liraglutide,
while ratings of nausea correlated with decreased brain activation. Thus they
interpreted that presence of GLP1 receptors was reported for 1st time in human
brains. They also saw that liraglutide alters brain activity related to highly
desirable food cues. Thus their data pointed to a central mechanism
contributing to or underlying the effects of liraglutide on metabolism and
weight loss. More studies would be required to confirm and extend these
findings in larger samples of diabetic individuals and or with the higher doses
of liraglutide (3 mg) that had been recently approved for obesity treatment
[41].
To study if obese individuals with more
components of metabolic syndrome (MetS) and/or prediabetes showed activation of
brain centres in response to food cues, Farr et al. [42] examined prediabetes
(n=26) vs. obese non-diabetics (n=11), using fMRI. They also did regression
analysis on the basis of the number of MetS components/subject. Obese
individuals with prediabetes had reduced activation of the reward related
putamen in the fasting state and reduced activation of the salience and
reward-related insula after eating. Obese individuals with components of MetS
showed reduced activation of putamen in the fasting state. All these
activations remain significant when correlated with BMI, Waist Circumference
(WC), HbA1c and gender. Decreased activation in reward related brain areas
between obese individuals is more pronounced in subjects with prediabetes and
MetS. Greater prospective studies are required to quantify their contribution
to the development of prediabetes/MetS and to study if these conditions might
predispose to exacerbation of obesity along with development of comorbidities
over time [42]. Similarly in another study Farr et al. [43] showed that obese
individuals having type2 diabetes mellitus (T2DM) showed less activation of
the salience and reward related insula
while fasting and increased activation of amygdala to highly desirable foods
after a meal. Thus these findings in T2DM suggested a persistence of difference
between obese versus non-obese individuals. Future larger studies can confirm
the differential activation between lean and obese individuals with and without
DM [43].
Walnuts have specific properties like high
alpha-linoleic acid (ALA) content that might add to the obesity and T2DM
reducing properties of walnuts [44,45]. The group of Mantzoros, Farr et al.
[46] showed walnuts increased satiety and fullness [45]. Previously walnuts
were shown to improve memory and increase hippocampal N-methyl-D-aspartate
(NMDA) receptors in rats which suggested they might have effects on the brain
[46]. Thus Farr et al. [47] performed a randomized, placebo controlled trial of
10patients who received living in a controlled environment either walnuts as
smoothie or placebo for 5 days each, separated by a wash out period of one
month using fMRI. Walnut consumption reduced feelings of hunger and appetite
assessed using visual analog scales and increased activation of the right
insula to highly desirable foods. Thus concluding that walnut consumption might
increase salience and cognitive control processing of highly desirable food
cues, the beneficial metabolic effects observed [47].
CONCLUSION
Thus trying to study higher brain centres
communicating with the hypothalamus further helps besides studying the
alteration in hormones interacting with hypothalamus for homeostatic states
helps us in further understanding how BS, the most effective treatment till
date for human obesity works. We have been trying to study how anti-obesity
drugs orally can be utilized in better getting treatment without BS [48], which
is not only costly and not without risks besides limited indications for
BMI>35 kg/m2 with comorbidities or >=40 kg/m2
[49,50]. Further studying these interactions with other drugs that have been
approved for obesity like lorcaserin, GLP1 Analogues like liraglutide and other
drugs give us a better insight besides changes in this connectivity in obese
and diabetic patients has helped to understand how some naturally occurring
products like walnuts might be utilized for better control of T2DM. Further
doing these studies in thulakoids will better help in understanding how these
naturally occurring alkaloids found in spinach can get utilized for obesity
therapy [51].
1. Sparks
PJ, Bollinger M (2011) A demographic profile of obesity in the adult and
veterans population in 2008. Popul Res Policy Rev 30: 211-233.
2. Kochar
Kaur K, Allahbadia GN, Singh M (2013) Current management of obesity in an
infertile female: Recent advantages and future prospective drugs. J Pharm Nutr
Sci 3: 1-13.
3. Kochar
Kaur K, Allahbadia GN, Singh M (2016) An update on etiopathogenesis and management
of obesity. Obes Control Ther 3: 1-17.
4. Kochar
Kaur K, Allahbadia GN, Singh M (2018) Current advances in pathogenesis in
obesity: Impact of hypothalamic gliosis. J Obes Weight Loss 3.
5. Figelewicz
DP (2003) Adiposity signals and food reward: Expanding the CNS roles of insulin
and leptin. Am J Physiol Regul Integr Comp Physiol 284: R882-892.
6. Farr
OM, Li CR, Mantzoros CS (2016) Central nervous system regulation of eating:
Insights from human brain imaging. Metabolism 65: 699-713.
7. Volkow
ND, Wise RA (2005) How can drug addiction help us understand obesity? Nat
Neurosci 8: 555-560.
8. Volkow
ND, Wang GJ, Fowler JS, Tomasi D, Baler R (2012) Food and drug reward:
Overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci
11: 1-24.
9. Volkow
ND, Wang GJ, Tomasi D, Baler RD (2013) Obesity and addiction; Neurobiological
overlaps. Obes Rev 14: 2-18.
10. Volkow
ND, Wang GJ, Tomasi GJ, Baler RD (2013) The addictive dimensionality of
obesity. Biol Psychiatry 73: 811-18.
11. Volkow
ND, Wang GJ, Telang F, Fowler JS, Thanos PK, et al. (2008) Low dopamine
striatal D2 receptors are associated with prefrontal metabolism in obese
subjects: Possible contributing factors. Neuroimage 42: 1537-1543.
12. Michaelides
M, Thanos PK, Volkow ND, Wang GK (2012) Translational neuroimaging in drug
addiction and obesity. ILAR Jr 53: 59-68.
13. Spice
E, Spoor S, Bohon C, Veldhuizen MG, Small DM (2008) Relation of rewards from
food intake to obesity: A functional magnetic resonance imaging study. J Abnorm
Psychol 117: 924-935.
14. O’Doherty
JP, Deichmann R, Crichley HD, Dolan RJ (2002) Neural responses during
anticipation of a primary taste reward. Neuron 33: 815-826.
15. Small
DM, Veldhuizen MG, Felsted J, Mark YE, McGlose F (2008) Separable substrates
for anticipatory and consummatory food chemosensation. Neuron 57: 786-797.
16. Stoeckel
LE, Weller RE, Cook EW 3rd, Twieg DB, Knowlton RC, et al. (2008)
Widespread reward system activation in obese women in response to pictures of
high calorie foods. Neuroimage 41: 636-647.
17. Holsen
LM, Zarcone JR, Brooks MG, Thompson TI, Ahluwalia JS, et al. (2006) Neural
mechanisms underlying hyperphagia in Pradder–Willi syndrome. Obesity (Silver
Spring) 14: 1028-1037.
18. Boutelle
KN, Wierenga CE, Bischoff-Grether A, Melrose AJ, Greneso-Stevens E, et al.
(2015) Increased brain response to appetitive tastes in the insula and amygdale
in obese compared with healthy weight children when sated. Int J Obes (Lond)
39: 620-628.
19. Jastreboff
AM, Lacadie C, Seo D, Kubat J, Van Name MA, et al. (2014) Leptin is associated
with exaggerated brain reward and emotion responses to food images in
adolescent obesity. Diabetes Care 37: 3061-3068.
20. Mehta
S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, et al. (2012) Regional brain
response to visual food cues is a marker of satiety that predicts food choice.
Am J Clin Nutr 96: 989-999.
21. Sun
X, Kroemer NB, Veldhuizen MG, Babbs AE, De Araujo IE, et al. (2015) Basolateral
amygdale response to food cues in the absence of hunger is associated with
weight gain susceptibility. J Neurosci 35: 7964-7976.
22. Ulrich-Lai
YM, Christiansen AM, Wang X, Song S, Herman JP (2015) Statistical modeling
implicates neuroanatomical circuit mediating stress relief by comfort food.
Brain Struct Funct 221: 3141-3156.
23. Martin
AA, Davidson TL (2014) Human cognitive function and the obesogenic environment.
Physiol Behav 136:185-193.
24. Parent
MB, Darling JN, Henerson YO (2014) Remembering to eat: Hippocampal regulation
of meal onset. Am J Physiol Regul Integr Comp Physiol 306: 701-713.
25. Milanski
M, Degasperi G, Coope A, Moran J, Cintra DE, et al. (2009) Saturated fatty
acids produce an inflammatory response predominantly through the activation of
tlr4 signaling in hypothalamus: Implications for the pathogenesis of obesity. J
Neurosci 29: 359-370.
26. Thaler
JP, Choi SJ, Schwartz MW, Wise BE (2010) Hypothalamic inflammation and energy
homeostasis: Resolving the paradox. Front Neuroendocrinol 31: 79-84.
27. Corbetta
M, Kincade JM, Schulman GL (2002) Neural systems for visual orienting and their
relationship to working memory. J Cogn Neurosci 14: 508-523.
28. Corbetta
M, Shulman GL (2002) Control of goal directed and stimulus driven attention in
the brain. Nat Rev Neurosci 3: 201-215.
29. Arora
AR (2011) From reactive to proactive and selective control: Developing a richer
model for stopping inappropriate responses. Biol Psychiatry 69: 55-68.
30. Chapman
CD, Benedict C, Brooke SJ, Schioth HB (2012) Lifestyle determinants of drive to
eat: A meta-analysis. Am J Clin Nutr 96: 492-497.
31. Volkow
ND, Wang GJ, Fowler JS, Telang F (2008) Overlapping neuronal circuits in
addiction and obesity: Evidence of systems pathology. Philos Trans R Soc Lond B
Biol Sci 363: 3191-3200.
32. Volkow
ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, et al. (2009) Inverse
association between BMI and prefrontal metabolic activity in healthy adults.
Obesity (Silver Spring) 17: 60-65.
33. Abaramowski
D, Rigo M, Duc D, Hoyer D, Staulenbel M (1995) Localization of the 5-hydroxy
tryptamine 2C receptor protein in human and rat brain using specific antisera.
Neuropharmacology 34: 1635-1645.
34. Li G,
Ji G, Hu Y, Liu L, Jin Q, et al. (2018) Reduced plasma ghrelin concentrations
are associated with decreased brain reactivity to food cues after laparoscopic
sleeve gastrectomy. Psychoneiroendocrinology 100: 229-236.
35. Zhang
Y, Ji G, Li G, Hu Y, Liu L, et al. (2018) Ghrelin reductions following
bariatric surgery were associated with decreased resting state in the
hippocampus. Int J Obes (Lond).
36. Liu
L, Ji G, Li G, Hu Y, Jin Q, et al. (2018) Structural changes in brain regions
involved I executive control and self-referential processing after sleeve
gastrectomy in obese patients. Brain Imaging Behav.
37. Li G,
Ji G, Hu Y, Xu M, Jin Q, et al. (2018) Bariatric surgery in obese patients
reduced resting connectivity of brain regions involved with self-referential
processing. Hum Brain Mapp 39: 4755-4765.
38. Hoffman
BJ, Mezey E (1989) Distribution of serotonin 5HT1C receptor MRNA in adult rat
brain. FEBS Lett 247: 453-462.
39. Mengod
G, Nguuyen H, Le H, Waeber C, Palacios JM, et al. (1990) The distribution and
cellular localization of the serotonin 1C receptor MRNA in the rodent brain
examined by in situ hybridization histochemistry. Comparison with receptor
binding distribution. Neuroscience 35: 577-591.
40. Farr
OM, Upadhyay J, Gavreli A, Camp M, Spyrou N, et al. (2016) Lorcaserin:
Administration decreases activation of brain centres in response to food cues
and these emotion – and Salience – related changes correlate with weight loss
effects: A 4 week long randomized, placebo controlled, double blind clinical
trial. Diabetes 65: 2943-2953.
41. Farr
OM, Sofopoulous M, Tsoukas MA, Dincer F, Thakkar B, et al. (2016) GLP1
receptors exist in the parietal cortex, hypothalamus and medulla of the human
brains and the GLP1 analogue liraglutide alters brain activity related to
highly desirable food cues in individuals with diabetes: A crossover,
randomized, placebo-controlled trial. Doabetologia 59: 954-965.
42. Farr
OM, Mantzotos CS (2017) Obese individuals with more components of the metabolic
syndrome and/or prediabetes demonstrate decreased activation of reward-related
brain centers in response to food cues in both the fed and fasted states. A
preliminary FMRI study. Int J Obes (Lond) 41: 471-474.
43. Farr
OM, Mantzotos CS (2018) Obese individuals with type 2 diabetes demonstrated
decreased activation of the salience-related insula and increased activation of
the emotion-related amygdale to visual food cues compared to non-obese
individuals with diabetes: A preliminary study. Diabetes Obes Metab 20:
2500-2503.
44. Aronis
KN, Vamvini MT, Chamberland JP, Sweeney LL, Brennan AM, et al. (2012) Short
term walnut consumption increases circulating adiponectin A concentrations, but
does not affect markers of inflammation or vascular injury in obese humans with
the metabolic syndrome: Data from a double blinded, randomized, placebo
controlled study. Metab Clin Exp 61: 577-582.
45. Brennan
AM, Sweeney LL, Liu X, Mantzotos CS (2010) Walnut consumption increases
satiation but has no effect on insulin resistance or the metabolic profile over
a 4 day period. Obesity (Silver Spring) 18: 1176-1182.
46. Hicylimaz
H, Vural H, Delibas N, Sutcu R, Gultken F, et al. (2014) The effects of walnut
supplementation on hippocampal NMDA receptor subunits NR2A and NR2B of rats.
Nutr Neurosci 20: 203-208.
47. Farr
OM, Tuccinardi D, Upadhyay J, Oussaada SM, Mantzotos CS (2018) Walnut
consumption increases activation of the insula to highly desirable food cues: A
randomized, double-blind. Placebo-controlled crossover FMRI study. Diabetes
Obes Metab 20: 173-177.
48. Kochar
Kaur K, Allahbadia GN, Singh M (2018) An update on bariatric surgery with long
term efficacy and its utilization for medical therapy development from the
different mechanism of action and other short comes to be outcome. BAOJ Surg 4:
038.
49. Kochar
Kaur K, Allahbadia GN, Singh M (2016) further update on the management of
obesity with emphasis on genetic perspective. BAOJ Obes Weight Manage 3: 1-11.
50. Kochar
Kaur K, Allahbadia GN, Singh M (2018) Existing and prospective pathways for
intervention in treatment of obesity in a novel way - A review. MOJ Drug Des
Develop Ther 2: 94-104.
51. Kochar
Kaur K, Allahbadia GN, Singh M (2018) Can thylakoids replace bariatric surgery
for long term maintenance of weight loss in obesity giving a more physiological
approach. Obes Control Ther 5: 1-10.
52. Zheng
H, Berthoud R (2008) Neural systems controlling the drive to eat: Mind versus
metabolism. Physiology (Bethesda) 23: 75-83.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- BioMed Research Journal (ISSN:2578-8892)