2242
Views & Citations1242
Likes & Shares
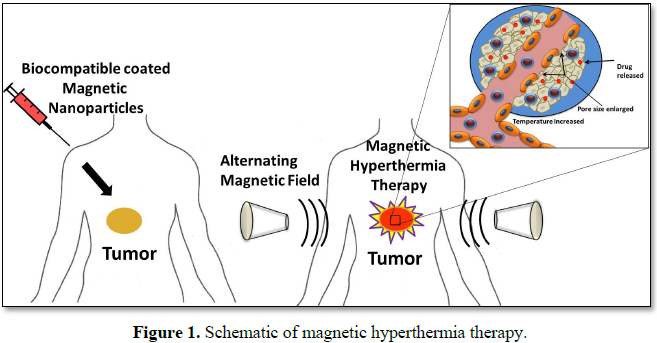
Nanotechnology provides a novel and original solution with magnetic hyperthermia; it is promising approach towards adjuvant cancer therapy. The use of magnetic nanoparticles to remotely induce local heat when alternating magnetic field (AMF) is applied, provoking a temperature increase in those tissues and organs where the tumorous cells are present, appears to be promising since it can lead to increased life expectancy in patients. Magnetic nanoparticle hyperthermia (MNP) is also being studied as a catalyst to conventional chemotherapy and radiation therapy. In this review, we elucidated the Fe3O4/γ-Fe2O3 magnetic nanoparticle coated with different biocompatible material and thermally capable as well as targeting agent has been described in order to reduce the proliferation pace on cancerous tumor.
Keywords: Magnetic nanoparticle, Magnetic nanoparticle hyperthermia (MNP)
INTRODUCTION
Cancer has become one of the major public health concerns in our modern society, resulting in the death of more than 8.8 million people every year across the world in 2015 [1]. Magnetic hyperthermia treatment (MHT) is one of the promising non-invasive approaches for thermal activation therapy on cancerous tumors. Upon exposure to external alternating current (AC) magnetic fields, MNPs can generate heat through the oscillation of their magnetic moments. MHT can then kill cancer cells by elevating their temperature to 40°C-45°C with minimal injury to normal cells [2]. Magnetic Hyperthermia based heating systems could be employed either for the controlled release of a drug from caged drug delivery systems or the killing of tumor cells or both. Magnetic Hyperthermia internalized in tumor cells would result in intracellular hyperthermia under an alternating current (AC) magnetic field due to hysteresis loss and consequent enhancement in the efficacy of magnetic hyperthermia based therapy [3,4]. Hence, application of magnetic hyperthermia provides a combinatorial therapeutic approach for cancer therapy, employing the advantages of a nanoparticles-based targeted drug delivery and the hyperthermia modality of cancer therapy [5].
VARIOUS BIOCOMPATIBLE MATERIAL COATED MAGNETIC NANOPARTICLES
For hyperthermia, large variety of magnetic nanoparticles, magnetite (Fe3O4) and maghemite (γ-Fe2O3) are most widely used magnetic system for hyperthermia applications because of their selective heating capacity without damaging healthy tissues, superior bio-compatibility, ease of synthesis and long term stability [2]. Hergt et al. [6] injected 100 mg dextran coated magnetic nanoparticles into the tail vein of Sprague Dawley rats, treated with AC magnetic field (12 min, 450 kHz, unknown field and SAR). According to authors’ considerable the tumor shrinkage and tissue necrosis was observed. Lao et al. [7] reported that the localized heat was achieved by subjecting (PVA+Fe3O4) ferrogel to alternating magnetic field and maximum temperature of 43°C was 2.5 wt% Fe3O4 concentrations. The time taken was ~5 min under field amplitude of 1.7 kA/m and frequency of 375 kHz. He also showed maximum temperature can be easily adjusted by changing the Fe3O4 magnetic field strength so as to meet different requirement of various cancer or tumor cells. Kim et al. [8] reported the comparison of chitosan-coated magnetic nanoparticle with uncoated and starch-coated magnetic nanoparticles targeting to carcinoma cells in hyperthermia. The chitosan-coated magnetic nanoparticles generated a higher ∆T of 23°C under an AC magnetic field than the starch coated magnetic nanoparticles and the capturing rate of the magnetic nanoparticles was also 96% under an external magnetic field
CONCLUSION
Advancement of nanotechnology in the field of cancer treatment has increased tremendously. Various material coated magnetic nanoparticle (Fe3O4/γ-Fe2O3) exhibit properties like good biocompatibility, low toxicity, ease of synthesis, surface functionalization and high specific absorption rate value which make them the most promising candidate for magnetic hyperthermia. Optimization in nanoparticle size, particle size distribution and frequency of AC magnetic field can lead to increase in efficacy of magnetic hyperthermia treatment. High cost, complicated process and harmful side effects of at present available cancer treatments can be replaced by Fe3O4/γ-Fe2O3 based magnetic hyperthermia.
1. Pinank K, Kaushik B, Shaym V, Amrutiya M, Faldu N, et al. (2016). Revolutionary therapies and manipulation of nanoparticles to cure cancer. J Bioelectron Nanotechnol 1: 1-5.
2. Darwish MSA, Nguyen NHA, Ševců A, Stibor I, Smoukov SK (2016) Dual-modality self-heating and antibacterial polymer-coated nanoparticles for magnetic hyperthermia. Mater Sci Eng C 63: 88-95.
3. Pala J, Mehta H, Mandaliya R, Moradiya M, Savaliya CR, et al. (2017) Tailoring of magnetic hyperthermia treatment using super paramagnetic iron oxide nanoparticles. MOJ Biol Med 2: 174-175.
4. Markna JH, Raval CS, Shah NA (2014) Current aspects of nanomedicine for cancer. Res Rev J Oncol Hematol 3.
5. Hildebrandt B, Wust P, Ahlers ODA, Sreenivasa G, Kerner T, et al. (2002) The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43: 33-56.
6. Hergt R, Hiergeist R, Zeisberger M, Schuler D, Heyen U, et al. (2005) Magnetic properties of bacterial magnetosomes as potential diagnostic and therapeutic tools. J Magn Mater 293: 80-86.
7. Lao LL, Ramanujan RV (2004) Magnetic and hydrogel composite materials for hyperthermia application. J Mater Sci Med 15: 1061-1064.
8. Kim DH, Kim KN, Kim KM, Lee YK (2009) Targeting to carcinoma cells with chitosan- and starch-coated magnetic nanoparticles for magnetic hyperthermia. J Biomed Mater Res 88A: 1-11.
9. Ghosh R, Pradhan L, Devi YP, Meena SS, Tewari R, et al. (2011) Induction heating studies of Fe3O4 magnetic nanoparticles capped with oleic acid and polyethylene glycol for hyperthermia. J Mater Chem 21: 133-188.
10. Tomitaka A, Yamada T, Takemura Y (2012) Magnetic nanoparticle hyperthermia using pluronic-coated Fe3O4 nanoparticles: An in vitro study. J Nanomater 2012: 1-5.
11. Lartigue L, Innocenti C, Kalaivani T, Awwad A, del Mar Sanchez Duque M, et al. (2011) Water-dispersible sugar-coated iron oxide nanoparticles. An evaluation of their relaxometric and magnetic hyperthermia properties. J Am Chem Soc 133: 10459-10472.
12. Iqbal Y, Bae H, Rhee I, Hong S (2016) Intensive analysis of core-shell silica-coated iron-oxide nanoparticles for magnetic hyperthermia. J Nanosci Nanotechnol 16: 11862-11867.
13. Soleymani M, Edrissi M (2016) Synthesis of bilayer surfactant coated magnetic nanoparticles and their application for magnetic fluid hyperthermia. J Dispers Sci Technol 37: 1793-1798.