974
Views & Citations10
Likes & Shares
Peripheral nerve injury may result from acute trauma or iatrogenic
causes. The emergence of improved
imaging techniques including high resolution ultrasound, MR neurography, and
diffusion tensor imaging, enables more accurate assessment of peripheral nerve
injury. We review two cases of
iatrogenic peripheral nerve injury and the manner in which peripheral nerve
surgeons may utilize multimodality perioperative imaging to guide management.
Keywords: Neurography,
Ultrasound, Neurotisation, Diffusion tensor, Tractography
Abbreviations: EMG:
Electromyography; MR: Magnetic Resonance; DTI: Diffusion Tensor Imaging; US:
Ultrasound
INTRODUCTION
Injury to
peripheral nerves is increasingly recognized as a source of morbidity in the
traumatized patient. One study from a
level I trauma center demonstrated a 2.8% prevalence of peripheral nerve injury
in its trauma population. The radial
nerve and peroneal nerves were the most frequently injured peripheral nerves in
the upper and lower extremities respectively [1]. Although the frequency of iatrogenic
peripheral nerve injury has not been well characterized, functional limb
impairment, such as foot drop in our cases, can be quite disabling. The Seddon and Sunderland classification
schemes have been long used to characterize peripheral nerve injury. The relative simplicity of the three category
Seddon scheme is useful to peripheral nerve surgeons as it correlates well with
the necessity for surgical management.
Grade 1 injury, or neurapraxia, in the Seddon system represents
conduction block secondary to demyelination at the site of injury and
corresponds to grade 1 injury in the Sunderland classification. Conduction is maintained distal and proximal
to the site of injury and therefore neurapraxia is typically reversible. Grade
2 injury, or axonotmesis, represents axonal loss with preservation of
surrounding connective tissue layers and corresponds to grade 2 injury
according to Sunderland. Recovery is
possible through axonal regeneration, a process that occurs at approximately 1
mm per day. The severest form of
peripheral nerve injury in the Seddon classification system is neurotmesis,
which involves disruption of myelin and surrounding connective tissue
elements. Frank discontinuity of the
nerve or intervening scar tissue prevents axonal regeneration and surgical
intervention is required for recovery of function [2]. The Sunderland scheme further subdivides
neurotmesis into three categories. Grade 3 injury involves disruption of the
endoneurial tubes while Grade 4 injuries extend to the perineurium and may
result in a neuroma-in-continuity [3]. A neuroma-in-continuity represents the
disorganized attempt of an injured but uninterrupted nerve to unite its
fascicles at the site of injury with the distal uninjured nerve.
It may also result from
lower grade injuries [4]. Grade 5
injury, according to Sunderland, involves the epineurium and frank disruption
of the nerve, often resulting in formation of a terminal neuroma. As in the Seddon classification system,
grades 4 and 5, and often grade 3 injuries, require surgical repair for recovery
of function [3]. Recent advances in
imaging, including magnetic resonance imaging (MRI) and ultrasound, enable
clinicians to accurately characterize the location, nature and extent of
peripheral nerve injury noninvasively, thus expediting the surgical plan. In cases of higher grade injury, the gap
between a severed nerve can be determined and nerve avulsion injuries can be
identified. In addition, advances in
surgical technique afford the opportunity for improved functional outcome. We
present two cases of iatrogenic peroneal nerve injury and the relevant imaging
studies utilized to more precisely characterize the pathologic anatomy and help
the treating peripheral nerve surgeon guide surgical management. In one case, the injured nerve was surgically
repaired with preoperative and intraoperative imaging providing useful
information. The other case was deemed non-operable.
Case 1
History
and Examination: The patient is a 60 year-old man
who sustained a right hamstring tendon injury during a fall. He underwent surgical repair at a separate institution
approximately one month later and was noted to have right foot drop
postoperatively. An iatrogenic nerve injury was suspected and the patient was
referred to our center for further care 9 months after the initial injury.
EMG studies demonstrated
findings most consistent with a right sciatic mononeuropathy between the
surgical scar in the upper thigh and the semitendinosus muscle. The peroneal division was primarily affected
with no recruitment of the tibialis anterior or peroneus longus muscles and
poor recruitment of the biceps short head muscle compared to the
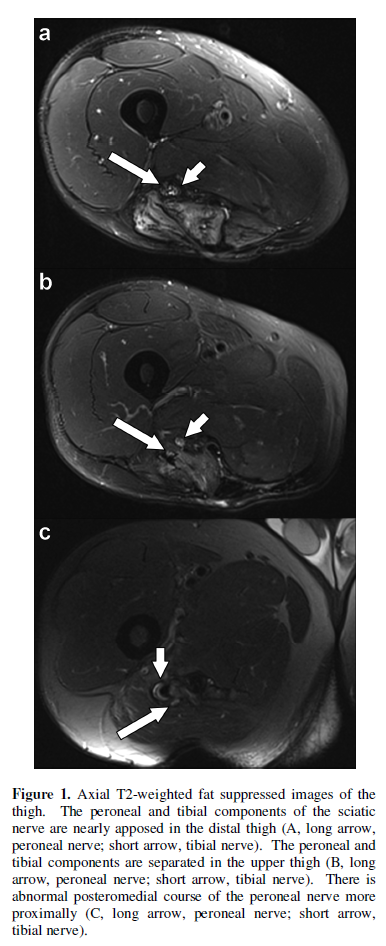
semitendinosus. MR neurography was performed on a 3T MRI scanner (MAGNETOM
Skyra, Siemens, Erlangen, Germany). It demonstrated abnormal separation of the
peroneal and tibial components of the sciatic nerve in the proximal thigh over
a distance of approximately 8 cm (Figure
1a, 1b). The peroneal component demonstrated an unusual course
posteromedial to the tibial component and appeared to terminate in the region
of the ischial tuberosity (Figure 1c).
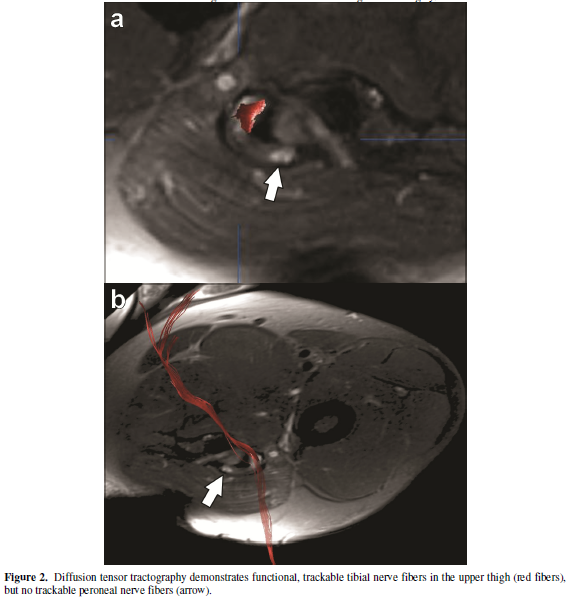
Further analysis with diffusion tensor imaging (DTI) demonstrated evidence of
functioning axons in the tibial component of the sciatic nerve but no fibers
were detected in the nonfunctioning peroneal component distal to the point of
presumed suture repair (Figure 2a, 2b).
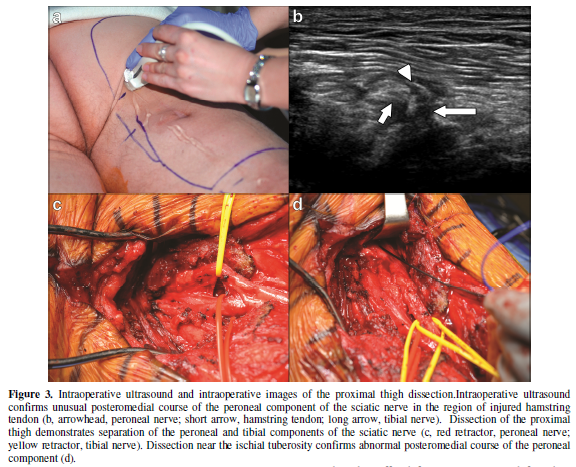
Operation: Ultrasound was performed intraoperatively using an iU22
ultrasound machine with a high frequency, 12MHz transducer (Philips Medical
Systems, Bothel, WA). The course of the
right sciatic nerve was delineated (Figure
3a). It was noted to have an unusual
twisted configuration in the proximal thigh/buttocks junction. An incision was
made along the course of the sciatic nerve using ultrasound guidance. Leads were placed in the tibial and peroneal
nerve-supplied muscles to monitor sensory and motor function throughout the
surgery.
Stimulation of the tibial
component of the sciatic nerve above the level of the popliteal fossa evoked
expected responses in the tibial nerve-supplied muscles and unexpected small
responses in the peroneus longus and tibialis anterior muscles. Stimulation of
the peroneal component at this level elicited no muscle response.
Proximal dissection of the
sciatic nerve confirmed the preoperative imaging findings. The tibial and peroneal components of the
sciatic nerve were identified as distinct structures in the proximal thigh (Figure 3b). Dissection of the proximal tibial component
was relatively routine. However, the peroneal component was scarred, requiring
circumferential neurolysis to expose the nerve.
The proximal peroneal component was then identified coursing
posteromedial to the tibial component, as suggested on MR neurography,
deviating from its normal lateral location (Figure 3c). The nerve was
sutured to scar and muscle near the ischial tuberosity(Figure 3d). More proximal dissection to the level of the sciatic
notch did not reveal a proximal peroneal nerve stump. At this point,
stimulation of the proximal tibial component of the sciatic nerve evoked
responses in the tibialis anterior and peroneus longus, suggesting a distal
anastomosis between the two components of the sciatic nerve. Expected tibial
nerve-supplied muscles also demonstrated a vigorous response.
Since a proximal stump
could not be identified, the patient had essentially sustained an avulsion injury
of the peroneal nerve. A decision was
made to perform end-to-side neurotisation of the distal peroneal nerve stump to
the side of the tibial nerve. The
distal, avulsed segment of the peroneal nerve was cut in the distal mid thigh
in a fish mouth configuration. The
tibial component was examined under magnification. Stimulation of a posterolateral region of the
nerve evoked motor responses in the peroneus longus and tibialis anterior
muscles. The epineurium in this region
was incised and the fishmouthed end of the peroneal component was sutured to
the side of the tibial component.
Case 2
History
and Examination: The patient is a 54-year-old
female with history of right hamstring injury 4 years prior to presentation.
Surgical repair was attempted shortly after the injury, but she suffered from
severe pain and foot drop postoperatively. Subsequent pain management and
physical therapy resulted in no significant improvement. Physical examination
revealed 0/5 strength for right foot eversion and toe dorsiflexion consistent
with a complete foot drop. There was
also diminished sensation along the dorsum of the foot. An old posterior right buttock incision was
identified.
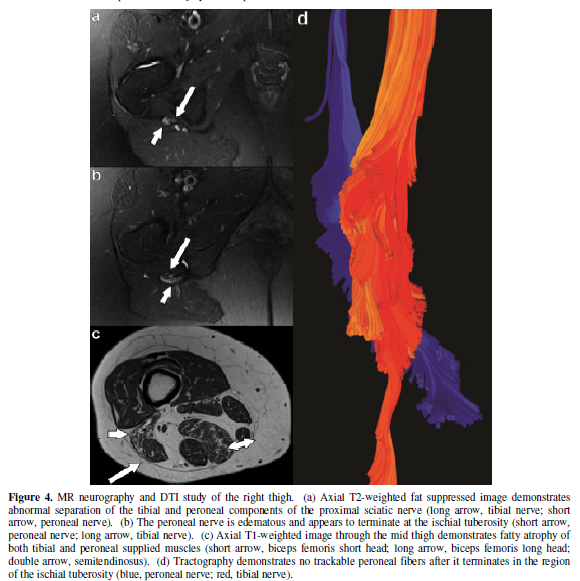
Imaging: MR neurography demonstrated separation of the tibial and
peroneal components of the proximal sciatic nerve after exiting the sciatic
notch (Figure 4a). The lateral, peroneal component was edematous
and enhancing. It was noted to course
posteromedially in the region of prior hamstring tendon repair where it
appeared to terminate (Figure 4b).There
was fatty atrophy of the biceps femoris short head muscle compatible with
injury to the peroneal component of the sciatic nerve. Probable tibial nerve injury was also
demonstrated as evidenced by fatty atrophy of the biceps femoris long head,
semimembranosus, and semitendinosus muscles (Figure 4c). Diffusion tensor
imaging confirmed abnormal separation of the proximal sciatic nerve into its
peroneal and tibial components. No trackable peroneal fibers were identified
distal to the ischial tuberosity, where the peroneal nerve appeared to
terminate, possibly having been sutured to the ischial tuberosity (Figure 4d).
Management: The chronic nature of the injury precluded surgical repair in
this case. Implantation of a sciatic
nerve stimulator was discussed as a tool for pain management, however the
patient opted for continued medical pain management.
DISCUSSION
One surgical approach to
peripheral nerve injury is neurorrhaphy, whereby distal and proximal nerve
stumps are directly anastomosed. A large
gap between nerve stumps precludes neurorrhaphy and the gap may be bridged by
an interposition nerve graft or conduit.
More severe injury such as nerve root avulsion may be addressed with
nerve root reimplantation, nerve transfer, or neurotisation. Neurotisation
procedures are well described in the setting of brachial plexus avulsion
injuries. The goal of neurotisation is to decrease the distance between the
target muscle and regenerating axons, thus decreasing the time to potential
recovery. During neurotisation, the distal portion of an injured nerve is
sutured to an intact, uninjured nerve. Successful recovery of target muscle
function has been reported with side-to-side, end-to-side, and end-to-end
techniques [5]. A number of findings are important in determining the
management of patients with peripheral nerve injuries, including the site and
degree of injury as well as its chronicity.
Imaging can be helpful to better characterize these injuries and provide
peripheral nerve surgeons with valuable information to better plan appropriate
management and possible surgical intervention.
Preoperative MR neurography,
DTI and intraoperative ultrasound can be used in conjunction with
intraoperative electrical stimulation to direct surgical management.
MR neurography utilizes
high signal-to-noise, high spatial resolution T1-weighted nonfat-suppressed and
heavily T2-weighted fat-suppressed sequences as well as isotropic 3D images to
evaluate the course, caliber, and fascicular architecture of nerves. Peripheral nerve edema, abnormal
fasciculation, aberrant course of a nerve, and frank disruption of a nerve can
all be assessed with MR neurography (6).
Sunderland Grades 1-3 nerve injury may result in diffuse nerve swelling
without focal enlargement. Signal
changes in the target muscle secondary to denervation may be seen with Grade 2
and Grade 3 injuries, although distinguishing between these grades is not
possible on imaging as the endonuerium is below the resolution of current MRI
techniques. This is of doubtful clinical significance because these lower grade
injuries are typically treated medically. MR neurography can be helpful in
assessing grade 4 or 5 injuries to ensure timely surgical intervention.
Neuroma-in-continuity or frank disruption of a nerve, including the gap between
its proximal and distal stumps, can be accurately characterized with MR
neurography [3]. Limitations of MR
neurography primarily relate to the limits of its spatial resolution and field
signal inhomogeneity, which may obscure the detection of subtle peripheral
nerve abnormalities.
Du and colleagues
demonstrated that MR neurography provided additional diagnostic information in
45% of patients with spinal and peripheral nerve disorders, beyond the
information provided by electromyography and nerve conduction studies [7]. In case #1, MR neurography yielded a more
precise anatomic characterization of the injury than preoperative EMG studies
which were only able to localize the injury to somewhere in the upper thigh. Abnormal morphology of the proximal right sciatic
nerve and the aberrant course of its peroneal component were MR neurography
findings corroborated intraoperatively.
In case #2 MR neurography provided anatomic correlation for the
patient’s clinically apparent foot drop.
Associated muscle fatty atrophy in the peroneal and tibial nerve
distributions confirmed the irreversible nature of the injury and helped the
treating peripheral nerve surgeon determine that surgical management would not
likely be helpful to this patient.
MR neurography may also provide
functional information by utilizing diffusion tensor imaging. Various quantitative parameters can be
obtained from this technique including fractional anisotropy, axial
diffusivity, radial diffusivity, and apparent diffusion coefficient. These parameters have been shown to be
reproducible in the evaluation of peripheral nerves [8]. It has been shown that
axial diffusivity correlates with axon integrity while fractional anisotropy
reflects myelin sheath integrity [9].
DTI can be used to develop tractograms which represent a 3-dimensional
graphical depiction of a nerve and its course. It must be noted that DTI and
tractography are extremely labor intensive techniques and the selection of
mathematical parameters such as fractional anisotropy and diffusivity can
greatly impact the accuracy of tractograms.
Selection of strict parameters may result in the false identification of
peripheral nerve disruption due to exclusion of intact fascicles. Conversely, overly inclusive parameters may
result in the false interpretation of intact fascicles when in fact none exist.
The quality of this technique also greatly varies with magnetic field strength,
offering much greater spatial resolution and decreased imaging time with 3
Tesla magnets. It should be emphasized
that tractograms provide functional information and not the anatomic
characterization of individual nerve fibers [10].
DTI in case #1 demonstrated
an intact tibial component of the sciatic nerve and disruption of the peroneal
component, which corroborated findings on EMG and intraoperative electrical
stimulation of the proximal sciatic nerve.
It did not identify the unexpected distal anastomosis between the tibial
and peroneal components of the sciatic nerve identified during intraoperative
electrical stimulation. This finding may
have been below the threshold of included parameters. Alternatively, scarring
or inflammation may have altered diffusivity.
In case #2, the DTI study demonstrated no trackable peroneal fibers
distal to the ischial tuberosity where the peroneal nerve appeared to terminate
(Figure 4d). This was consistent with the patient’s
clinically apparent complete foot drop.
Ultrasound may provide
additional preoperative and intraoperative evaluation to guide surgical
management. It offers the advantages of
being dynamic, cost effective, and fast.
The depiction of nerve pathology by high-resolution ultrasound parallels
that of MR neurography, including abnormal caliber, fascicular architecture,
course, or discontinuity of a nerve [11]. In addition, intraoperative
ultrasound can be used to map the location of peripheral nerves to guide
surgical approaches. Sonographic
evaluation of peripheral nerves may be limited when imaging deep structures. The nerve of interest may also be obscured by
bone, gas, and surgical material such as sutures.
CONCLUSION
We demonstrate the utility
of a multimodality imaging approach in the assessment of patients with
peripheral nerve trauma and the synergistic value of preoperative and
intraoperative imaging in a patient deemed to be a surgical candidate. Image-based anatomic and functional
characterization of the patient’s injury serve not only to confirm findings
suggested by clinical and electrophysiologic assessment, but may also to help
expedite a precise and well informed surgical approach. In other patients, such
as in our second case, the imaging findings of chronic nerve injury can help
the peripheral nerve surgeon deem that medical management rather than surgical
intervention would provide a better result.
Therefore, imaging may add significant value for the surgeon and
ultimately improve outcomes for peripheral nerve trauma patients.
- Nobe J, Munro CA, Prasad VS, Midha R (1998) Analysis of
upper and lower extremity peripheral nerve injuries in a population of
patients with multiple injuries. J Trauma 45: 116-122.
- Grant, GA, Goodkin R, Kliot M (1999) Evaluation and
Surgical Management of Peripheral Nerve Problems. Neurosurgery 44:
825-839.
- Chhabra A, Ahlawat S, Andreseik G (2014) Peripheral nerve
injury grading simplified on MR Neurography: As referenced to Seddon and
Sunderland classifications. Indian J Radiol Imag 24 : 217-224.
- Chhabra A, Williams EH, Wang KC, Dellon AL, Carrino JA (2010) MR neurography of neuromas related to nerve injury and entrapment with surgical correlation. AJNR 31: 1363-1368.
- Simon NG, Spinner RJ, Kline DG, Kliot M (2016) Advances
in the neurolgocical and neurosurgical management of peripheral nerve
trauma. J Neurol Neurosurg Psychiatry 87: 198-208
- Chhabra A, Andreisek G, Soldatos T, Wang KC, Flammang
AJ, et al. (2011) MR Neurography: past, present, future. AJR 197: 583-591.
- Du R, Auguste, KI, Chin CT, Engstom JW, Weinstein PR
(2010) Magnetic resonance reurography for the evaluation of peripheral
nerve, brachial plexus, and nerve root disorders. J Neurosurgery 112: 362-371.
- Simon NG, Lagopoulos J, Gallagher T, Kliot M, Kierman MC
(2016) Peripheral nerve diffusion tensor imaging is reliable and
reproducible. J Mag Reson Imag 43:
962-969.
- Heckel A, Weiler M, Xia A, Ruetters M, Pham M, et al.
(2015) Peripheral nerve diffusion tensor imaging: assessment of axon and
myelin sheath integrity PLoS One 10.
- SantarelliX, GarbinG, UkmarM, Longo R (2010) Dependence
of the fractional anisotropy in cervical spine from the number of
diffusion gradients, repeated acquisition and voxel size. Magn Reson Imag
28: 70–76.
- Yablon CM, Hammer MR, Morag Y, Brandon CJ, Fessell, DP
(2016) US of the peripheral nerves of the lower extremity: a landmark
approach. Radiographics 36.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)