633
Views & Citations10
Likes & Shares
Introduction: PONV is the most distressing complication of
anesthesia and surgery. PONV occurs in about 30% of all surgical patients and
in 70-80% of high risk patients. Several receptors like dopaminergic,
cholinergic, histaminic and serotonergic are involved in pathophysiology of
vomiting. Among them selective 5-HT3 receptor antagonist like palonosetron is
now a 1st line of option because of its effectiveness and general lack of
adverse reactions.
Aim: To compare the efficacy, duration of action
and side effects of palonosetron and ondansetron as a prophylactic regimens for
prevention of PONV in patients undergoing MRM under general anesthesia.
Materials and methods: After obtaining institutional
review board approval and written informed consent 100 adult patients of ASA
grade I and II undergoing modified radical mastectomy were randomly divided
into two different groups (50 patients in each group).
Group P:
Palonosteron 0.075 mg (prior to induction). Group O: Ondansetron 8 mg (prior to
induction).
All
patients were assessed for the incidence of nausea, retching, vomiting, total
PONV, complete response, requirement of rescue antiemetic and presence of
adverse effects from 0-24 h at 3 h interval.
Results: The incidence of Nausea, retching and
vomiting was lower in the palonosetron group compared to ondansetron group
during all study period however this difference was not statistically
significant (P>0.05). However the incidence of total PONV was significantly
less in group P than group O during 0-24 h with P<0.05. Complete response
was significantly more in the palonosetron group (60%) compared with the
ondansetron group (26%) (P<0.05). 16 patients in group O required rescue
anti-emetics as compared to 6 patients in group P during 0-24 h time interval
and the difference was statistically significant (P<0.05). Incidence of
adverse effects was comparable and no significant difference was observed
between two groups with P value>0.05.
Conclusion: Palonosetron is more effective in preventing
PONV with fewer requirements of recue antiemetic in comparison to ondansetron
in patients undergoing MRM under GA.
Keywords: Palonosetron, Ondansetron, Postoperative
nausea and vomiting, Modified radical mastectomy
INTRODUCTION
Post-operative
nausea and vomiting (PONV), defined as nausea and or vomiting occurring within
24 h after surgery [1-4]. It is described as “The big little problem” and from
the patients perspective, PONV is the most distressing complication of
anesthesia and surgery [5]. Patients reports that avoidance of PONV is of
greater concern than avoidance of postoperative pain [6,7].
PONV not only
causes pain, but also leads to dehydration, anxiety, acid base and electrolyte
imbalance and wound dehiscence [8]. Hence PONV represents a major challenge in
the practice of modern anesthesia. PONV occurs in about 30% of all surgical
patients and in 70-80% of high risk patients [9,10].
The genesis of PONV is multifactorial and
result from activation of 4 vomiting centers: the vestibular system, the CTZ,
the GI vagal system, and the cortical center and is influenced by patient,
surgery and anesthesia related factors. Several receptors like dopaminergic,
cholinergic, histaminic and serotonergic are involved in pathophysiology of
vomiting [1]. Among them selective 5-HT3 receptor antagonist is now a 1st line
of option because of its effectiveness and general lack of adverse reactions
[11,12].
Palonosetron is a newer 5-HT3 receptor
antagonist approved by USFDA for prevention of PONV in 2008 [13]. Its unique
pharmacodynamics mechanism of allosteric binding and positive co-operativity
trigger internalization, result in persistent inhibition and long duration of
action [14].
The present prospective randomized study was
aimed to compare the efficacy, duration of action, and side effects of
palonosetron and ondansetron as prophylactic regimens for prevention of PONV in
patients undergoing MRM under general anesthesia.
AIMS AND OBJECTIVE
Primary
To compare the
efficacy of IV palonosetron with IV ondansetron in preventing PONV during 1st
24 h following MRM.
Secondary
·
To compare the need for rescue antiemetic in both
the groups.
·
To compare the side effects of study drugs in both
the groups.
MATERIALS AND METHODS
Selection of patients
100 adult patients
ranging from 18 years to 60 years undergoing modified radical mastectomy were
selected for the study. Only patients belonging to ASA I and ASA II were
selected for study. Patients were assessed adequately in the pre-operative
period. Thorough history and clinical examination, investigations were
conducted and analyzed.
Exclusion criteria
·
History of motion sickness and previous history of
PONV.
·
Full stomach.
·
Gastro esophageal reflux disease.
·
Pregnant and menstruating women.
·
Those who had taken antiemetic medication within 24
h.
·
Known history of allergy to any study drug.
Method
After obtaining
institutional review board approval, written informed consent was obtained from
100 adult patients undergoing modified radical mastectomy and were randomly
divided into two different groups (50 patients in each group).
Group P:
Palonosteron 0.075 mg (prior to induction). Group O: Ondansetron 8 mg (prior to
induction).
Patients were kept
nil by mouth for at least 8 h before surgery. Patients were pre-medicated with
Tab. Lorazepam night before surgery. Anesthetic techniques were identical in
all patients. Anesthesia was induced with IV Inj. Glycopyrrolate (4 mcg/kg),
Inj. Thiopentone Sodium (5-7 mg/kg), Inj. Fentanyl (1-2 mcg/kg). Tracheal
intubation was facilitated with IV Inj. Vecuronium Bromide (0.1 mg/kg) and with
cuffed endotracheal tube of appropriate size. Anesthesia was maintained with O2
(50%) + N2O (50%) + Sevoflurane (0.4% to 1.0%). Muscle relaxation
was provided by Inj. Vecuronium Bromide (0.1 mg/kg) IV. Ventilation was
controlled and adjusted to maintain an end tidal concentration of CO2
between 30 and 40 mm of Hg.
Intra operatively
patients were monitored with pulse oximetry, ECG, non-invasive blood pressure
measurement and end tidal CO2 concentration.
All patients were
reversed at the end of surgery with inj. glycopyrrolate and Inj. neostigmine
and extubated after return of pharyngeal and laryngeal reflexes.
Postoperative assessment: All
patients were assessed for the incidence of nausea, retching, vomiting, total
PONV, complete response, requirement of rescue antiemetic and presence of
adverse effects from 0-24 h at 3 h interval.
Rescue antiemetic: Inj.
Metoclopramide 10 mg IV was given on patient demand or 2 or more episodes
nausea, vomiting or retching was recorded.
Postoperative pain assessment: At the
surgical site was assessed by using VAS scale (0-No Pain to 10 Most Severe
Pain).
Analgesic: All the patients
were given diclofenac sodium 1.5 mg/kg intramuscularly (max. 75 mg) as
analgesic at 8 hourly intervals after surgery or earlier if they demanded pain
relief.
STATISTICAL ANALYSIS
Data were analyzed
using computer statistical software system Graph Pad. Categorical variables
between the study groups were assessed by Z-test. Similarly, comparisons among
two groups involving quantitative variables were assessed by the Student’s
t-test. Differences between groups were declared as statistically significant
at P<0.05.
OBSERVATION AND RESULTS
The following observation and results were noted in
a comparative study between ondansetron and palonosetron to prevent
post-operative nausea and vomiting in 100 patients undergoing modified radical
mastectomy.
The incidence of
Nausea, retching and vomiting was lower in the palonosetron group compared to
ondansetron group during all study period (P>0.05). But this difference was
not statistically significant. However the incidence of total PONV was
significantly less in group P than group O during 0-24 h with P<0.05 (Table
2).
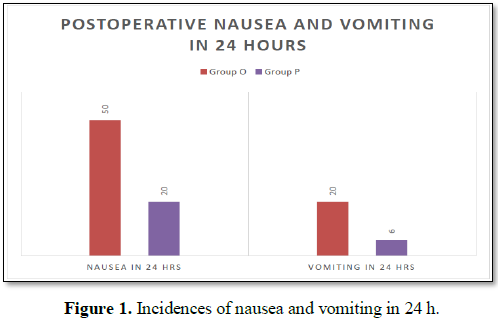
In group O, 50% patients while in group P, 20% of
patients experienced nausea during 24 h postoperatively and this difference was
statistically significant (P<0.05). Also incidence of vomiting in 24 h
postoperatively was significantly less in palonosetron group (6%) compared to
ondansetron group (20%) (P<0.05) (Table 3).
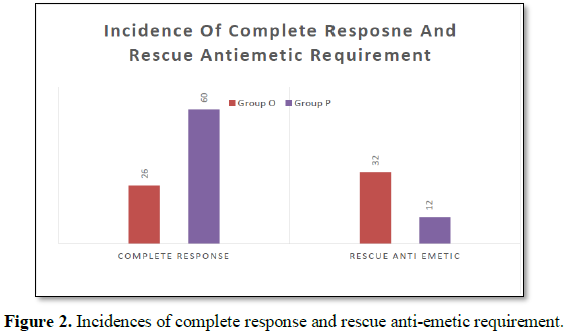
Complete response was significantly more in the
palonosetron group (60%) compared with the ondansetron group (26%) (P<0.05).
16 patients in group O required rescue anti-emetics as compared to 6 patients
in group P during 0-24 h time interval and the difference was statistically
significant (P<0.05) (Table 4 and Figure 1).
The Heart rate and mean arterial pressure between
the study groups had no significant difference during 0-24 h postoperatively
with P>0.05 (Table 5 and Figure 2).
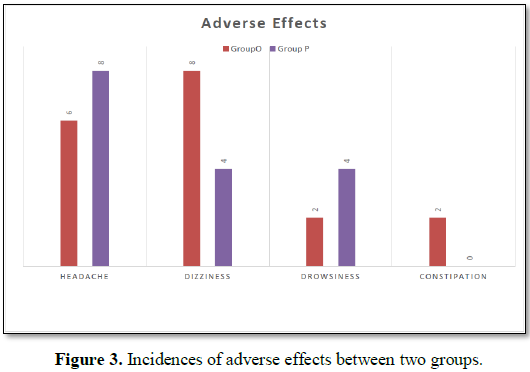
Incidence of adverse effects was comparable and no
significant difference was observed between two groups with P value>0.05 (Table
6 and Figure 3).
DISCUSSION
Post-operative nausea and vomiting is most common and distressing complication after surgery and anesthesia. PONV not only causes pain but also leads to anxiety, dehydration, electrolyte and acid-base imbalances, aspiration pneumonia and wound dehiscence [8]. It is a leading cause of delayed postoperative recovery and discharge.
The genesis of PONV is multifactorial involving operative, anesthetic and patient specific factors. Apfel et al. [15] stated that female, a history of PONV or motion sickness, nonsmoker and postoperative opioid use were the more important risk factors and each additional risk factor increased the PONV incidence rate to 21, 39, 61 and 79%. Several receptors like dopaminergic, serotonergic, cholinergic and histaminic are involved in the pathophysiology of vomiting. The use of anti-emetics, either alone or in combination remains the mainstay of PONV management. Drugs used include anti-cholinergic, dopamine antagonists, anti-histaminic, steroids and selective 5-HT3 receptors antagonists. Among them serotonergic receptor antagonists are 1st line drug of PONV prophylaxis because of its effectiveness, more safety and favorable side effects [11,12].
In 1990s 5-HT3 receptor antagonists was heralded as the major advance in prophylaxis against PONV and are routinely used now a days to prevent PONV as they lack major adverse effects [16-18.] Ondansetron, granisetron, dolasetron, topisetron and palonosetron are currently available 5-HT3 receptor antagonists [17].
Ondansetron, selective 5-HT3 receptor antagonists is considered as the first 5-HT3 receptor antagonists highly effective antiemetic that has been used for both prevention and treatment of PONV [19]. Its antiemetic effect is stronger than its anti-nausea effect. It has a short half-life of 3-5 h [20]. It’s being routinely used either alone or in combination with other drugs in day care surgeries for PONV prophylaxis because of its lower cost.
Palonosetron is a new, potent 2nd generation 5-HT3receptor antagonists with unique structural, pharmacological and clinical characteristics. Its allosteric binding creates a conformational change in serotonin receptor so that serotonin binding is indirectly inhibited [21]. Consequently, palonosetron has higher affinity with 5-HT3 receptors, which ultimately leads to greater potency and longer duration of (20%) action of 40 h in comparison with standard 5-HT3 antagonists [10,22].
Our study was done to compare the efficacy of palonosetron 0.075 mg and ondansetron 8 mg for prevention of PONV in patients undergoing MRM under general anesthesia. Study drug was administered prior to induction of anesthesia based on the hypothesis that greater antiemetic effect of drugs is seen if we block the CTZ before the arrival of emetic stimuli associated with anesthesia and surgery.
Honkavaara et al. [23] in their study concludes that ondansetron 8 mg was not superior to 4 mg in preventing PONV and the need for rescue antiemetic. In our study we selected ondansetron 8 mg on the basis of Paventi et al. [24] dose ranging study of ondansetron in which they concluded that single dose of ondansetron 8 mg was more effective than ondansetron 4 mg and is the minimum effective dose in the prevention of PONV. A study done by Tramer et al. [25] also in view of that ondansetron 8 mg is the optimal dose for the prevention of PONV.
Candiotti et al. [26] evaluated the three different single IV doses of palonosetron (0.025 mg, 0.05 mg and 0.075 mg) compared with placebo for the prevention of PONV in patients at risk for nausea and or vomiting. They observed a linear trend in efficacy with increasing doses, with the highest dose (0.075 mg) of palonosetron demonstrating a statistically significant effect compared with placebo over the first 24 h. In addition Kovac et al. [27] also in his study compared palonosetron in doses of 0.025 mg, 0.05 mg and 0.075 mg. They found that lower doses were not as effective as palonosteron 0.075 mg, which significantly reduced the severity of nausea and delayed the time to emesis. US FDA also approved 0.075 mg as the minimum effective dose of palonosetron for PONV prophylaxis [26,28]. Therefore we chose palonosetron 0.075 mg in our study.
We did not include a control group receiving placebo as Aspinall and Goodman [29] have suggested that if effective drugs are available, placebo controlled trials maybe unethical.
In the present study both the groups were comparable with respect to age, sex, body weight and mean duration of surgical procedure (Table 1) with no statistical difference between two groups (P>0.05).
No major hemodynamic changes were observed in either group. Our observations were similar to the previous studies [30-32].
In our study, incidence of nausea, retching, vomiting and total PONV were observed during 0-3, 3-6, 6-9, 9-12 and 12 -24 h time interval postoperatively. We found that the incidence of nausea was 18% in group O and 8% in group P at 0-3 h. While during 3-6 h, it was 12% and 4% in group O and group P, respectively. At 6-9 h group O had 8% while group P had 2% of nausea. During 9-12 h it was 6% in group O compared to 4% in group P and at12-24 h 6% and 2% in group O and group P, respectively. While comparing incidence of vomiting, during 0-3 h it was 8% in group O while it was 4% in group P, at 3-6 h we found 6% and 2% as incidence of vomiting in group O and group P, respectively. During 6-9 h it was 2% in group O and 0% in group P, while at 9-12 h we found it was 4% and 0% in group O and group P and during 12-24 h both group O and P had 0% vomiting. While comparing the incidence of retching during 0-24 h it was found to be less in palonosetron group than ondansetron group. From the above findings it was clear that incidence of nausea, retching and vomiting was less in the palonosteron group during all the time periods compared to ondansetron group. Though the incidence was lower in the palonosetron group than ondansetron group, they were not statistically significant (P>0.05).
This is in accordance with Patel et al. [33] study, where they found the incidence of nausea during 0-2 (5.71% vs. 14.29%), 2-6 (5.71% vs. 14.29%) and 6-12 (0% vs. 8.57%) hours and the incidence of vomiting during 0-2 (2.86% vs. 11.43%), 2-6 (0% vs. 2.86%) and 6-12 (2.86% vs. 2.86%) hours’ time interval was less in the palonosetron group than ondansetron group but this difference was not statistically significant. Similar results were observed in Ahmed et al. [34] study where they studied the incidence of PONV in patients who were given either palonosteron or ondanserton for prophylaxis of PONV in middle ear surgery. They found incidence of nausea and vomiting was lower in palonosetron group as compared to ondansetron group but was not significantly different between the two groups.
In our study we found the overall incidence of nausea during 24 h time interval postoperatively was 50% in group O compared to 20% in group P and the difference was statistically significant with P=0.001. And the overall incidence of vomiting during 24 h postoperatively was 20% in ondansetron group and 6% in palonosteron group which was statistically significant (P=0.037). Bajwa et al. [35] found significantly higher incidence of nausea and vomiting (20% and 13.33%) in ondansetron group during 0-72 h in comparison to 6.67% and 3.33% in palonosteron group respectively (P<0.05). Similarly Taninder Singh et al. [36] found that the overall incidence of post-operative nausea in 24 h was 56.66% in patients among ondansetron group and 30% in patients of palonosetron group with statistically significant difference (p=0.037) between the two and the overall incidence of vomiting during 24 h was 20% in ondansetron group and 3.33% in palonosetron group (P=0.044). In the study conducted by Sarvesh et al. [37] the incidence of nausea during 0-24 h was 26.6% in ondansetron group and 8.9% in palonosetron group with significant difference between two (P=0.0005) and the incidence of vomiting during 24 h study period was 21.8% and 4% in group ondansetron and palonosetron, respectively, which was statistically significant with P value of 0.0001.
In case of breakthrough PONV, according to Guidelines from the Society for ambulatory anesthesia (SAMBA) it has been recommended that when PONV occurs after antiemetic prophylaxis, rescue drug used should be from a different class than one used for prophylaxis [42]. Candiotti et al. [43] in their study concluded that patients who failed ondansetron prophylaxis did not have a significant response to the same class of drug. In Bhalla et al. [44] study, the rescue antiemetic used was Inj. dexamethasone 8 mg IV as it has been recommended that patients should receive a rescue antiemetic drug from a different class of anti-emetics than one used for prophylaxis. They found that the need for rescue anti-emetics was significantly higher in patients receiving ondansetron (32%) as compared to palanosetron (16%). Based on the above results we used Inj. Metoclorpamide 10 mg as a rescue anti-emetics in our study if 2 or more episodes of vomiting occurs or on patient demand. In study conducted by Patel et al. [33], 5.71% of patients in palonosetron group and 23.53% patients in ondansetron group required rescue anti-emetic and this difference was statistically significant. Also in Singh et al (2014)[45] the need for rescue antiemetic was significantly more in ondansetron group (23.53%) than in the palonosetron group (5.71%). In our study patient requiring rescue anti-emetic was 32% in group O, while 12% in group P (P=0.0157) with statistically significant difference between the two and it was comparable to the above study findings.
In our study adverse effects with single IV dose of palonosetron and ondansetron were not clinically serious and there were no significant difference in the incidence of headache, dizziness or drowsiness between two groups. In the study done by Kim et al. [46] they did not find any significant difference in the incidence of side effects among two groups. Also in the study done by Laha et al. [30], Ahmed et al. [34] and Patel [33] found no significant differences in the side effect profile between the two groups confirming our findings.
CONCLUSION
In conclusion, the results of the present study clearly conveys that the palonosetron hydrochloride is more effective in preventing PONV with less requirement of recue anti-emetics in comparison to ondansetron hydrochloride in patients undergoing MRM under GA.
1.
Kovac AL (2000) Prevention and
treatment of postoperative nausea and vomiting. Drugs 59: 213-243.
2.
Watcha MF, White PF (1992)
Postoperative nausea and vomiting. Its etiology, treatment and prevention.
Anesthesiology 77: 162-184.
3.
Lerman J (1994) Surgical and
patient factors involved in postoperative nausea and vomiting. Br J Anesth 69:
24S-32S.
4.
Cohen MM, Duncan PG, DeBoer DP,
Tweed WA (1994) The postoperative interview: Assessing risk factors for nausea
and vomiting. Anesth Analg 78: 716.
5.
Kapur PA (1991) The big “little
problem”. Anesth Analg 73: 243-245.
6.
Orkin FK (1992) What do
patients want? Preference for immediate postoperative recovery. Anesth Analg
74: S225.
7.
Macario A, Weinger M, Carney S,
Kim A (1999) Which clinical anesthesia outcomes are important to avoid? The
perspective of patients. Anesth Analg 89: 652-658.
8.
Singh T, Shah N, Patel C,
Upadhayaya RM (2014) A comparative study of prophylactic ondansetron versus palonosetron
for post-operative nausea and vomiting in middle ear surgeries. Int J Biomed
Adv Res 5: 619622.
9.
Gan TJ (2002) Postoperative
nausea and vomiting – Can it
be eliminated? JAMA 287: 1233-1236.
10.
Sinclair DR, Chung F, Mezei G
(1999) Can postoperative nausea and vomiting be predicted? Anesthesiology 91: 109-118.
11.
Wallenborn J, Eberhart LH,
Kranke P (2009) Postoperative nausea and vomiting – What’s new in anti-emetic
pharmacotherapy? [Article in German]. Anasthesiol Intensivmed Notfallmed
Schmerzther 44: 296-304.
12.
Kloth DD (2009) New
pharmacologic findings for the treatment of PONV and PDNV. Am J Health Syst
Pharm 66: S11-S18.
13.
Ho KY, Gan TJ (2006)
Pharmacology, pharmacogenetics and clinical efficacy of 5 hydroxytryptamine
type 3 receptor antagonists for postoperative nausea and vomiting. Curr Opin
Anesthesiology 19: 606-611.
14.
Rojas C, Stathis M, Thomas
AG, Massuda EB, Alt J, et al. (2008) Palonosetron exhibits unique molecular
interactions with the 5HT3 receptor. Anesth Analg 107: 469-478.
15.
Apfel CC, Laara E, Koivuranta
M, Greim CA, Roewer N, et al. (1999) A simplified risk scores for predicting
postoperative nausea and vomiting: Conclusion from cross-validations between
two centres. Anesthesiology 91: 693-700.
16.
Paxton LD, McKay AC, Mirakhur
RK (1995) Prevention of nausea and vomiting after day case gynecological
laparoscopy. A comparison of ondansetron, droperidol, metoclopramide and
placebo. Anesthesia 50: 403-06.
17.
Craft TM, Upton PM (2001)
Anesthesia clinical aspects. 3rd Edn, pp: 279-281.
18.
Yoshitaka F, Hiroyoshi T,
Hideori T (1994) Optimal anti-emetic dose of granisetron for prevention of post-operative
nausea and vomiting. Can J Anesth 41: 94-97.
19.
Muchatuta NA, Paech MJ (2009) Management
of postoperative nausea and vomiting: Focus on palonosetron. Ther Clin Risk
Manag 5: 21-34.
20.
Blackwell CP, Harding SM (1989)
Clinical pharmacology of ondansetron. Eur J Cancer Clin Oncol 25: 21-27.
21.
Rojas C, Stathis M, Thomas AG,
Massuda EB, Alt J, et al. (2008) Palonosetron exhibits unique molecular
interactions with the 5HT3 receptor. Anesth Analg 107: 469-478.
22.
Wong EH, Clark R, Leung E
(1995) The interaction of RS 25259-197, a potent and selective antagonist, with
5-HT3 receptors, in vitro. Br J
Pharmacol 114: 851-859.
23.
Honkavaara P (1996) Effect of
ondansetron on nausea and vomiting after middle ear surgery during general
anesthesia. Br J Anesth 76: 316-318.
24.
Paventi S, Santevecchi A,
Ranieri R (2001) Efficacy of a single-dose ondansetron for preventing post-operative
nausea and vomiting after laparoscopic cholecystectomy with sevoflurane and
remifentanil infusion anesthesia. Eur Rev Med Pharmacol Sci 5: 59-63.
25.
Tramer MR, Reynolds DJ, Moore
RA, McQuay HJ (1997) Efficacy, dose response and safety of ondansetron in
prevention of postoperative nausea and vomiting: A quantitative systematic review
of randomized placebo controlled trials. Anesthesiology 87: 1277-1289.
26.
Candiotti KA, Kovac AL, Melson
TI, Clerici G, Joo Gan T, et al. (2008) A randomized, double-blind, study to
evaluate the efficacy and safety of 3 different doses of palonosetron versus
placebo in preventing postoperative and post-discharge nausea and vomiting.
Anesth Analg 107: 445-451.
27.
Kovac AL, Eberhart L, Kotarski
J, Clerici G, Apfel C, et al. (2008) A randomized, double-blind study to
evaluate the efficacy and safety of three different doses of palonosetron
versus placebo in preventing postoperative nausea and vomiting over a 72 h
period. Anesth Analg 107: 439-444.
28.
White PF, Song D, Abrao J,
Klein KW, Navarette B, et al. (2005) Effect of low-dose droperidol on the QT
interval during and after general anesthesia: A placebo-controlled study.
Anesthesiology 102: 1101-1105.
29.
Aspinall RL, Goodman NW (1995)
Denial of effective treatment and poor quality of clinical information in
placebo controlled trials of ondansetron for postoperative nausea and vomiting:
A review of published trials. Br Med J 311: 844-846.
30.
Laha B, Hazra A, Mallick S
(2013) Evaluation of anti-emetic effect of intravenous palonosetron versus
intravenous ondansetron in laparoscopic cholecystectomy: A randomized
controlled trial. Indian J Pharmacol 45: 24-29.
31.
Swaika S, Pal A, Chatterjee S,
Saha D, Dawar N, et al (2011) Ondansetron, ramosetron or palonosetron: Which is
a better choice of anti-emetic to prevent postoperative nausea and vomiting in
patients undergoing laparoscopic cholecystectomy? Anesth Essays Res 5.
32.
Nupur C, Shiv KR (2013)
Comparison between efficacy of palonosetron and ondansetron in postoperative
nausea and vomiting in middle ear surgery: A randomized double blind study. Int
J Pharm Biol Sci 4: 67-74.
33.
Patel A, Sangawar M,
Shelgaonkar V (2017) Palonosetron: A promising new prophylactic for PONV. Int J
Biomed Adv Res 8: 284-287.
34.
Ahmed El-Hamid AM, Othman MSK,
Afi EE (2014) Palonosetron versus ondansetron for prevention of postoperative
nausea and vomiting during middle ear surgery: A double-blind, randomized,
comparative trial. Ain-Shams J Anesthesiol 7: 309-313.
35.
Sukhminderjit SB, Sukhwinder
KB, Jasbir K, Veenita S, Amarjit S, et al. (2011) Palonosetron: A novel
approach to control postoperative nausea and vomiting in day care surgery.
Saudi J Anesth 5: 19-24.
36.
Singh T, Shah N, Patel C, Upadhayaya
RM (2014) A comparative study of prophylactic ondansetron versus palonosetron
for post-operative nausea and vomiting in middle ear surgeries. Int J Biol Adv
Res 12: 620-622.
37.
Sarvesh NK, Shivakumar PS
(2016) A comparative study of ondansetron and palonosetron as anti-emetics for
prevention of postoperative nausea and vomiting in patients undergoing
laparoscopic surgeries. J. Evol Med Dent Sci 5: 2430-2435.
38.
Park SK, Cho EJ (2011) A
randomized, double-blind trial of palonosetron compared with ondansetron in
preventing postoperative nausea and vomiting after gynecological laparoscopic
surgery. J Int Med Res 39: 399-407.
39.
Moon YE, Joo J, Kim JE, Lee Y
(2012) Anti-emetic effect of ondansetron and palonosetron in thyroidectomy: A
prospective, randomized, double blind study. Br J Anesth 108: 417-422.
40.
Sureshkumar K, Sivashanmugam A,
Murugan NG, Lazarus SP, Mukunthan MN, et al. (2016) Randomized controlled study
to compare the efficacy of intravenous palonosetron and intravenous ondansetron
in preventing post-operative nausea and vomiting in laparoscopic surgeries. Int
J Sci Stud 3: 288-294.
41.
Singh N, Raw BK, Kumar S,
Mishra MLS (2016) Palonosetron vs. ondansetron for prevention of postoperative
nausea and vomiting in patients undergoing laparoscopic cholecystectomy: A
comparative study. IOSR J Dent Med Sci 2: 45-49.
42.
Gan TJ, Diemunsch P, Habib AS,
Kovac A, Kranke P, et al. (2014) Consensus guidelines for the management of
postoperative nausea and vomiting. Anesth Analg 118: 85-113.
43.
Candiotti KA, Nhuch F, Kamat A,
Deepika K, Arheart KL, et al. (2007) Granisetron versus ondansetron treatment
for breakthrough postoperative nausea and vomiting after prophylactic
ondansetron failure: A pilot study. Anesth Analg 104: 1370-1373.
44.
Bhalla J, Baduni N, Bansal P
(2015) Comparison of palanosetron with ondansetron for postoperative nausea and
vomiting in patients undergoing laparoscopic cholecystectomy under general anesthesia.
J Minim Access Surg 11: 193-197.
45.
Singh T, Shah N, Patel C,
Upadhayaya RM (2014) A comparative study of prophylactic ondansetron versus
palonosetron for post-operative nausea and vomiting in middle ear surgeries.
Int J Biomed Adv Res 619622.
46.
Kim YY, Moon SY, Song DU, Lee KH, Song JW, et
al. (2013) Comparison of palonosetron with ondansetron in prevention of
postoperative nausea and vomiting in patients receiving intravenous
patient-controlled analgesia after gynecological laparoscopic surgery. Korean J
Anesthesiol 64: 122-126.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)