764
Views & Citations10
Likes & Shares
Tropical spastic
para paresis a disease of nervous system is caused by Human T lymphotrophic
virus type I thus also known as HTLV-I associated myelopathy and common among
female of age group 30-50 years in approximately 2-3% of HTLV-1 affected
person.
In spite advancement
in diagnostic procedure, i.e., CT scan, MRI its treatment with α-interferon,
steroid, antiviral drugs, neurovitamin supplementation, physiotherapy fails to
ensure cure or improve quality of life except transient pain relief with
analgesics and muscle relaxants, thus a therapeutic regime composite consisting
a proven herbal neurogenic been evaluated
Objective of study: To assess the herbal neurogenic and immune
boosting composite in ensuring clinical relief and improving quality of life in
patients deterred from various medi centres without any relief.
Materials and methods: 63 diagnosed and already treated cases of
tropical spastic para paresis attending at Centre For Critical Care National
Institute of Health and Research Warisaliganj (Nawada), Bihar been selected,
interrogated, examined clinically, assessed and analysed their previous
investigation reports, therapeutics taken and their effect.
Irrespective of
their clinical severity all patients were dvocated the prescribed regime and
were followed for post therapy 2 years for which patients been given a follow
up card to record the changes.
Results: 88.9% patients had grade I clinical response while rest 11.1% grade II
without any untoward effect or any withdrawal during post therapy 2 years
follow up.
Keywords: Tropical spastic para paresis, Human T lymphotrophic virus - type
I, CT, MRI, Herbal neurogenic, Quality of life
INTRODUCTION
Tropical spastic para paresis, a chronic and progressive clinical
condition affecting nervous system remained of obscure etiopathogenesis for
long but now a days an important association of this condition been established
between Human retrovirus (Human T cell lymphotropic virus type I) thus this
condition is also termed as HTLV1 associated myelopathy (HAM).
As per WHO estimate worldwide 10-20 million peoples are carrying HTLV1
and 5% of it are affected with TSP of age group 30-50 years [1-10].
TSP is very common in Latin America, the Caribbean Basin, sub-Saharan
Africa and Japan but these days incidence of this clinical state is increasing
even in India.
Common presentation of the clinical condition is [11-14]:
·
Gradual weakening and stiffening of
lower extremity
·
Radiating back pain down to legs
·
Burning and pricking sensation
(paraesthesia)
·
Urinary and bowel function disturbances
·
In male erectile dysfunction
·
Inflammatory skin condition like
dermatitis or psoriasis
The common mode of transmission of this virus is through [15,16]:
·
Breastfeeding
·
Sharing infected needles during intravenous drug use
·
Sexual activity
·
Blood transfusions
In spite of advancement in diagnostics (CT scan and MRI) and it’s
established etiopathogenesis till date no established therapeutic regime
ensured its reversal but only symptomatic relief, i.e., α-interferon,
intravenous immunoglobulin, antiviral drugs and muscle relaxants Tizanidine.
Signs and symptoms vary but may include slowly progressive weakness and
spasticity of one or both legs, exaggerated reflexes, muscle contractions in
the ankle and lower back pain. Other features may include urinary incontinence
and minor sensory changes, especially burning or prickling sensations and loss
of vibration sense.
Considering the poor quality of life with present therapeutics a clinical
study was planned to evaluate the clinical efficacy of proved neurogenic herbal
composite with neuro modulator at National Institute of Health and Research and
Centre for Research in Indigenous Medicine.
OBJECTIVE OF THE
STUDY
To evaluate he clinical efficacy and safety profile of herbal neurogenic
with neuromodulator in TSP.
MATERIALS AND METHODS
Duration of study
January 2014 to December 2018.
Materials
Patients of proved and treated cases of Tropical spastic Para paresis without any clinical response, attending at Centre for Critical Care, National Institute of Health and Research were considered for evaluation of the herbal neurogenic constituting therapeutic regime.
Methods
Patients of spastic para paresis diagnosed by myelogram, Computerized
Tomography (CT) and Magnetic Resonance Imaging (MRI) been interrogated
thoroughly for the onset, duration and evolution of the disease, family history
of neurological illness, history of extramarital sexual exposure, abortion,
blood transfusions, dietary with emphasis on strict vegetarianism, Lathyrus sativus, socio-economic status,
housing, sanitary conditions, treatment taken and their response. A detailed
general examination and a meticulous neurological assessment were done.
Patients were investigated
for hemoglobin concentration, total and differential leucocyte count,
erythrocyte sedimentation rate (ESR), peripheral smear, fasting and
postprandial blood sugar, renal and liver function tests and serological test
for syphilis. Common presentation of TSP can be summarized as (Box 2):
All patients underwent conventional myelography CT and MRI scans. The
serum samples of all the patients were tested for HTLV-1 antibodies by the
serodia technique.
All patients presenting with this crippling disease were advised and
administered the following therapeutic regime after due awareness counseling
and encouragement (Box 3):
Herbal composite NEUROVIT
Syr or Capsule constitutes - Cap 500 mg or Syr. 5 ml constitutes 100 mg each of
Acorus calamua (rhizome), Nardostachys jatamansi (Flower), Herpestis monnieri (leaf), Convolvulus pluricaulis (flower), Cassia acutifolia (seed).
Patients were assessed for improvement in tone and power of the muscle, tingling
and numbness, gait and autonomic function (passage of stool and urine) for
which patients were given a follow up card to mention date of achievement and
any untoward manifestation experienced. Patients were advised to visit the
center on any unusual manifestation or contact on helpline for needful
redresses.
To adjudge the safety profile of the regime practiced basic bio
parameters were repeated every month for first three month and then every 3
months.
Based on the clinical outcome and safety profile therapeutic response was
graded as (Box 4):
RESULTS
63 identified, diagnosed and treated patients of tropical spastic para
paresis considered for study were of age group 30-50 years and out of them
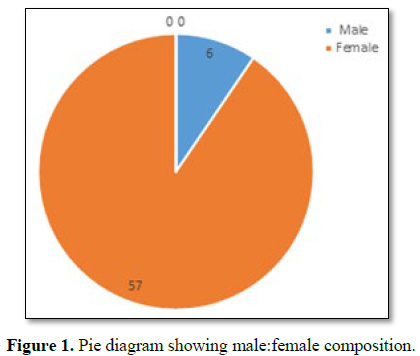
majority (30/63) were of age group 30-35 years with female dominance (Table
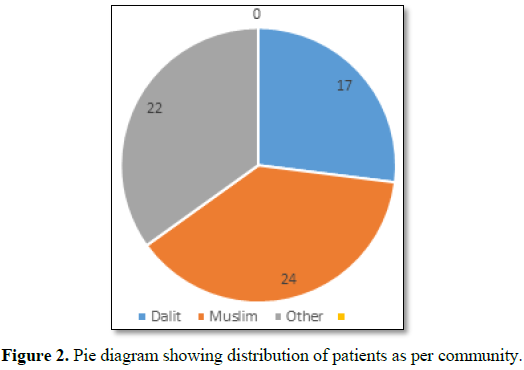
1 and Figure 1) and all were from rural background and community
representation was (Figure 2).
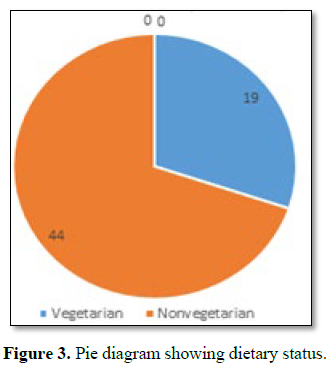
Out of all majorities were non vegetarian and non-had any history of
taking Lathyrus sativus (Figure 3).
The age of onset of clinical presentation varied from 20-40 years and
duration of illness from 1 year to 12 years (Figure 4).
Symptoms at the onset were difficulty in walking, stiffness of legs, back
pain, weakness of legs, leg pain and urinary discomfort while presenting
presentation at our center were disturbed gait, leg stiffness, back pain, leg
pain urinary discomfort, urinary retention, tingling and numbness, erectile
deficiency in male cases (Table 2).
No history of blood transfusion, abortion, delivery or surgery prior to
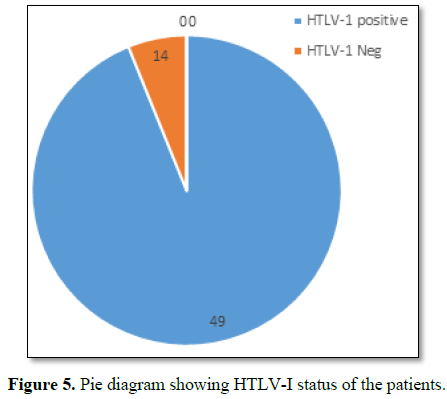
onset of the disease but serum samples revealed positive for HTLV-1 in 49 cases
out of 63. In addition all the bio parameters (hepatic, hematological and renal
profile remain normal) (Figure 5).
No patients were positive for tuberculosis, any sexually transmitted
disease, CT and MRI also shows normal in all the cases.
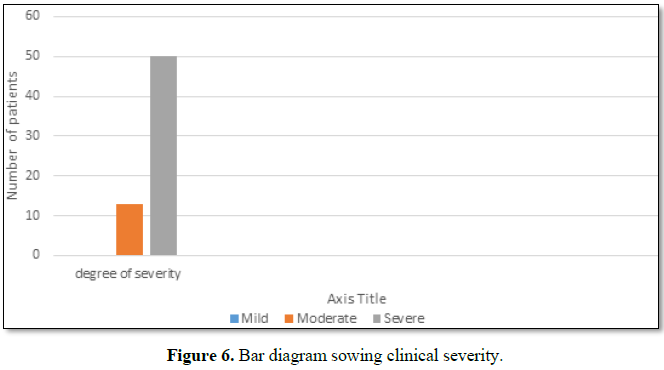
Out of 63 patients 13 were of moderate and 50 were of severe status (Figure
6).
Patients had taken treatment with α interferon, muscle relaxants,
neurovitamin supplementation at various medicare centers without any positive
therapeutic outcome (Table 3).
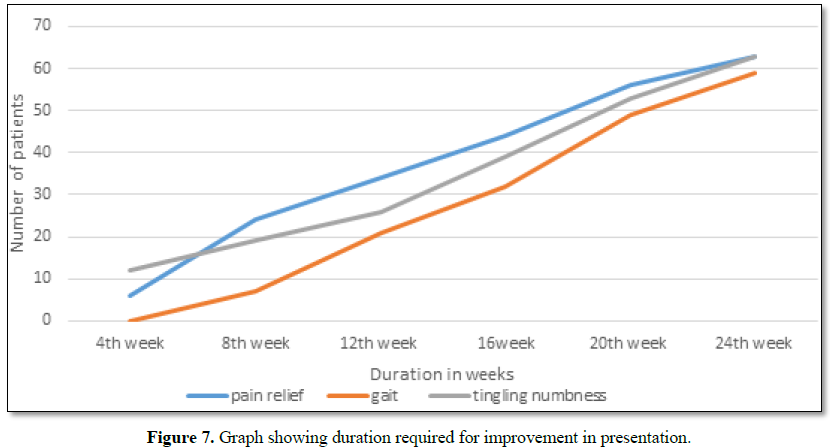
Symptomatic relief started from 4th week of therapy and by 24th week all had symptomatic relief (Figure 7).
The minimum and maximum duration of therapy required for complete
reversal of clinical presentation (both symptom and sign) is 9 months and 2
years, respectively.
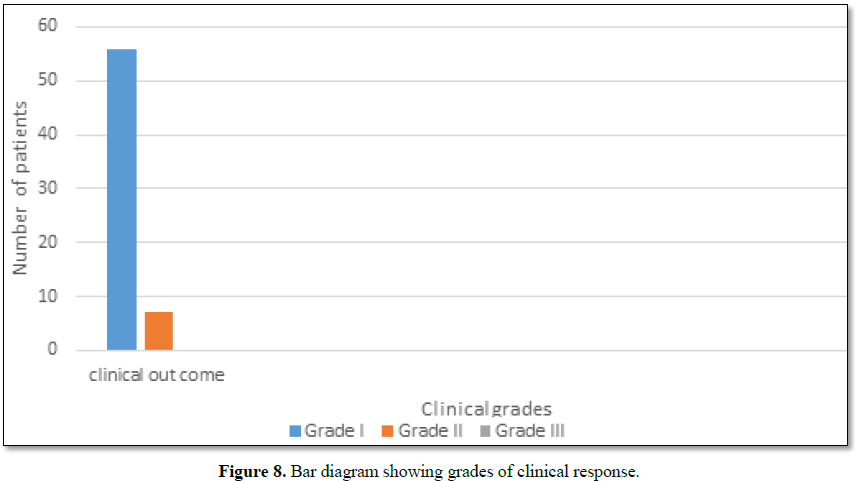
Out of all 56 patients achieved Grade I clinical improvement and 7 Grade
II (Figure 8).
No patients showed any adversity, recurrence of presentation or any
alteration in bio parameters in 2 years of post-therapy follow up (Table 4).
DISCUSSION
Tropical spastic para paresis is also common neurological disorder in
India though it’s a common in different parts of the world, i.e., including
Jamaica, Martinique, Seychelles, Colombia and Japan. Though it was considered
as a neurological disorder of obscure etiology but these days it is proved to
be caused by Human T Cell Lymphotropic virus type I (HTLV-I). In spite of
advancement in diagnostics like CT, MRI, CSF and Serum for HTLV-I antigen
[18,19], the therapeutics used, i.e., alpha interferon, muscles relaxant and
neurovitamin supplement [20-22] fails to ensure cure or improve quality of life
except transient symptomatic relief. Clinical supremacy in term of marked
improvement in pain, sensation and gait of the already treated patients with
other regime and achieving Grade I clinical response in 88.9% patients and
Grade II in rest 11.1%. No patients had any withdrawal or drug adversity in 2
years post therapy follow up.
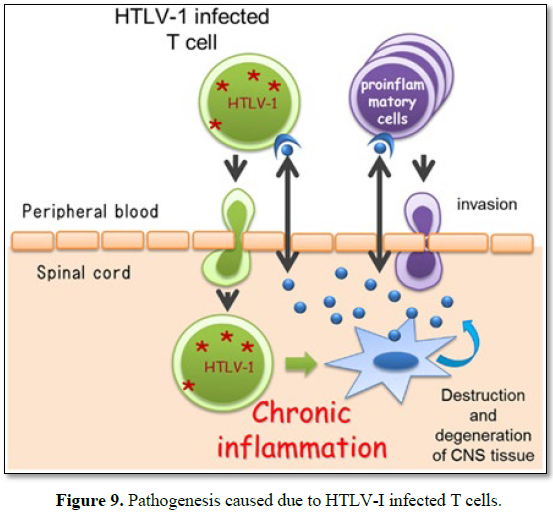
This clinical efficacy can be explained as (Figure 9):
Considering its pathogenesis and caused due to HTLV-I infected T cells.
Self-blood with Betamethasone intramuscular induces antibody formation
against the released toxin and ensure their neutralization while betamethasone
acting as anti-inflammatory reduces neural edema synergized by intravenous
calcium administration whose inclusion of one mole exit 2 mol of sodium acting
on sodium potassium ATPase pump and facilitate decrease in neural edema and
calcium ion improves neural conduction.
Methyl cobalamine, pyridoxine, niacin and pantothenic acid support neural
cells in its normal neural conduction and Neurovit a herbal composite by its
neurogenic activity helps in restoration of neural viability and vitality which
combinely ensure relief in pain ,neuropathic manifestation ,gait and autonomic
function and provide better quality of life to all.
CONCLUSION
Present regime constituting calcium gluconate intravenous, methyl cobalamine+Pyridoxin+Niacin intravenous, self-blood (2 ml) and Betamethasone 2 mg intramuscular, cap Cholecalciferol 60 K, syrup herbal neurotonic (Neurovit) proves worth in management of tropical spastic para paresis even in chronic and long term treated cases.
1.
World Health Organization (WHO) (1989) Human T
lymphotropic virus type 1, HTLV-1. Wkly Epidemiol Rec 64: 382-383.
2.
Orland JR, Engstrom J, Fridey J, Sacher RA, Smith
JW, Nass C, et al. (2003) Prevalence and clinical features of HTLV neurologic
disease in the HTLV outcomes study. Neurology 61: 1588-1594.
3.
Blattner WA, Gallo RC (1985) Epidemiology of human
retroviruses. Leuk Res 9: 697-698.
4.
Oomman A, Madhusoodanan M (2003) Tropical spastic
paraparesis in Kerala, South India. Neurol India 51: 493-496.
5.
Roman GC (1988) The neuroepidemiology of tropical
spastic paraparesis. Ann Neurol 23: 113-120.
6.
Arango C, Concho M, Zaninovic V, Biojor B, Rodgers
I, et al. (1988) Epidemiology of tropical spastic paraparesis in Colombia and
associated HTLV-1 infection. Ann Neurol 23: 161-165.
7.
Richardson JH, Newell AL, Newman PK, Mani KS, Rangan
G, et al. (1989) HTLV-1 and neurological disease in South India. Lancet i:
1079.
8.
Gessain A, Barin F, Vernant JC, Gout O, Calendar A,
et al. (1985) Antibodies to human T-lymphotropic virus type I in patients with
tropical spastic paraparesis. Lancet ii: 407-410.
9.
Rubin M (2016) Tropical spastic
paraparesis/HTLV-1-associated myelopathy (TSP/HAM). Merck Manual. Available at:
http://www.merckmanuals.com/professional/neurologic-disorders/spinal-cord-disorders/tropical-spastic-paraparesis-htlv-1%E2%80%93associated-myelopathy-tsp-ham
10.
(2017) Tropical spastic paraparesis information
page. National Institute of Neurological Disorders and Stroke. Available at: https://www.ninds.nih.gov/Disorders/All-Disorders/Tropical-Spastic-Paraparesis-Information-Page
11.
Iwasaki Y
(1990) Pathology of chronic myelopathy associated with HTLV-I infection
(HAM/TSP). J Neurol Sci 96: 103-123.
12.
Izumo S, Umehara F, Osame M (2000) HTLV-1 associated
myelopathy. Neuropathology 20: 565-568.
13.
Osame M (2002) Pathological mechanisms of human
T-cell lymphotropic virus type I-associated myelopathy (HAM/TSP). J Neurovirol
8: 359-364.
14.
Lezin A, Olindo S, Oliere S, Varrin-Doyer M, Marlin
R, et al. (2005) Human T lymphotropic virus type I (HTLV-I) proviral load in
cerebrospinal fluid: A new criterion for the diagnosis of HTLV-I-associated
myelopathy/tropical spastic paraparesis? J Infect Dis 191: 1830-1834.
15.
Matsuzaki T, Nakagawa M, Nagai M, Usuku K, Higuchi
I, et al. (2001) HTLV-I proviral load correlates with progression of motor
disability in HAM/TSP: Analysis of 239 HAM/TSP patients including 64 patients
followed up for 10 years. J Neurovirol 7: 228-234.
16.
De Castro-Costa CM, Araújo AQ, Barreto MM,
Takayanagui OM, Sohler MP, et al. (2006) Proposal for diagnostic criteria of
tropical spastic paraparesis/HTLV-I-associated myelopathy (TSP/HAM). AIDS Res
Hum Retroviruses 22: 931-935.
17.
Bagnato F, Butman JA, Mora CA, Gupta S, Yamano Y, et
al. (2005) Conventional magnetic resonance imaging features in patients with
tropical spastic paraparesis. J Neurovirol 11: 525-534.
18.
Scadden DT, Freedman AR, Robertson P (2016) Human
T-lymphotropic virus type I: Disease associations, diagnosis and treatment.
Waltham, MA: UpToDate. Available at: http://www.uptodate.com/contents/human-t-lymphotropic-virus-type-i-disease-associations-diagnosis-and-treatment
19.
Sandbrink F (2015) Tropical myeloneuropathies
treatment and management. Medscape Reference. Available at: http://emedicine.medscape.com/article/1166055-treatment
20.
Arimura K, Nakagawa M, Izumo S, Usuku K, Itoyama Y,
et al. (2007) Safety and efficacy of interferon-α in 167 patients with human T-cell
lymphotropic virus type 1-associated myelopathy. J Neurovirol 13: 364-372.
21.
Croda MG, de Oliveira AC, Vergara MP, Bonasser F,
Smid J, et al. (2008) Corticosteroid therapy in TSP/HAM patients: The results
from a 10 years open cohort. J Neurol Sci 269: 133-137.
22.
Taylor GP, Goon P, Furukawa Y, Green H, Barfield A,
et al. (2006) Zidovudine plus lamivudine in human T-lymphotropic virus
type-l-associated myelopathy: A randomised trial. Retrovirology 3: 63.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Spine Diseases
- Oncology Clinics and Research (ISSN: 2643-055X)
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Ophthalmology Clinics and Research (ISSN:2638-115X)