722
Views & Citations10
Likes & Shares
An association
between oral infections and systemic diseases has been suspected for centuries.
The effect of oral health on the rest of the human body was proposed by the
Assyrians in the seventh century BC. In the 18th century, a Pennsylvania
physician named Benjamin Rush was quoted as remarking that arthritis could be
treated in some people after they had infected teeth extracted. Over the past
decade, a growing body of scientific evidence suggests an exquisite association
between oral infection (e.g., viruses, bacteria, yeast) and systemic diseases (e.g.,
atherosclerosis, cardiovascular disease, cerebrovascular disease, prematurity
and low birth weight and pulmonary diseases and disorders) and also between
systemic diseases (e.g., arthritis, diabetes, HIV infection, and osteoporosis)
and oral, dental and craniofacial diseases and disorders.
Keywords: Periodontal regeneration, Critical issues, Periodontal
Abbreviations: GTR: Guided Tissue Regeneration; GBR: Guided
Bone Regeneration; BMP: Bone Morphogenetic Protein; PDL: Periodontal Ligament;
PRF: Platelet Rich Fibrin; HA: Hydroxyapatite; EMP: Enamel Matrix Proteins;
CBCT: Cone-Beam Computed Tomography
INTRODUCTION
Periodontitis is an infectious disease that causes destruction of the tooth-attachment apparatus, if left untreated, results in progressive attachment loss that may eventually lead to early tooth loss [1,2]. The goal of periodontal therapy is to restore periodontal tissues affected by disease to their original architectural form and function. This requires regeneration of the gingival connective tissues destroyed by inflammation, formation of cementum, restoration of lost bone, re-establishment of connective tissue fibers into previously diseased root surfaces [3]. The regenerative capacity of the periodontium rekindled the flame to research various techniques and materials for the same. For many decades, periodontists have been interested in regenerating tooth-supporting tissues destroyed by periodontal disease [4].
Conventional periodontal therapy consists of scaling, root planing, gingival curettage, gingivectomy and flap procedures of various types, including osseous surgery is effective in stabilizing periodontal status and maintaining periodontal health. Restitution of the periodontium to normal has often been an elusive, frustrating objective of periodontal therapy. This therapeutic modality has resulted primarily in repair rather than regeneration of the periodontium. Periodontal regeneration is unique because it involves both soft (gingival and periodontal ligament) and mineralized (bone and cementum) connective tissues. The healing of all periodontal components needs to be coordinated and integrated in order for regeneration to occur. Complete regeneration may be an unrealistic goal for many situations; due in part to the complexity of the biological events, factors and cells underlying successful periodontal regeneration [5,6].
There are various critical issues which act collaboratively as a road blocker in the path to attain complete regeneration and need to be addressed to achieve the objective well worth pursuing. The objective of this review is to bring the reader update regarding the critical issues in periodontal regeneration.
BIOLOGICAL FACTORS
Epithelial occlusion and promotion of progenitor cells
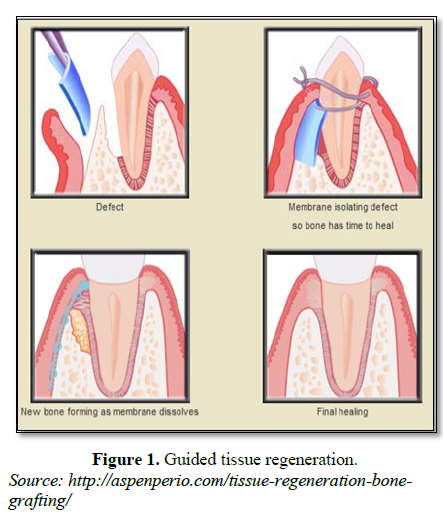
One of the crucial cellular events is recruitment of cells as the cell-type selection determines whether healing occurs by repair or regeneration. When the flap is replaced against the tooth after surgical procedure, the most aggressive tissue during the initial phases of wound healing is gingival epithelium which leads to repair with formation of long junctional epithelium which is a weaker union [4]. The critical issue is the regulation of timing of differentiation and maturation of all progenitor cells to restrain the epithelial cells from forming long junctional epithelium and simultaneously promoting selective population by regenerative cells to achieve true regeneration. In this context, clinical procedures and devices were envisioned to retard epithelial tissues from early access to the root surface. Since the pioneering guided tissue regeneration (GTR) experiments of just over 30 years ago, studies have repeatedly demonstrated that periodontal regeneration is biologically possible and clinically feasible [5]. However, GTR technique is sensitive and technically demanding. In addition, these modalities present with increased chair side time for the clinician and pose a financial burden for the patients (Figure 1).
The critical disadvantages of both polytetrafluoroethylene-based nonresorbable (e.g. second surgery) and resorbable membranes, mainly those based on collagen (e.g. insufficient mechanical properties, unpredictable degradation profiles), have led to studies of alternate membrane materials with the necessary mechanical, degradation and biological properties [7].
· E-spinning technique has demonstrated great potential for processing membranes for periodontal regeneration E-spinning is a particularly promising technique for synthesizing biomimetic nanomatrices, including membranes for GTR/Guided Bone Regeneration (GBR) applications [8]. The three-dimensional structure shown by these e-spun membranes, with a high surface area of improved hydrophilicity and wettability, endow the structure with mechanical support and regulate cell functions guiding new bone formation into the defect [7].
· Functionally graded and multi-layered membranes - The rationale of having a periodontal membrane with a graded-structure again relies on the principle that one can tailor the properties of the different layers to design a membrane that will retain its structural, dimensional and mechanical integrity long enough to enhance periodontal regeneration. The functionally graded membrane consists of a core-layer and two functional surface-layers interfacing bone (nanohydroxyapatite) and epithelial (metronidazole) tissues wherein the core layer comprises a neat poly(d,l-lactide-cocaprolactone) layer surrounded by two composite layers composed of a gelatin/polymer ternary blend [7].
i. Anti-bacterial properties: It is extremely important to control and/or reduce the bacterial contamination of the periodontal defect in order to enhance periodontal regeneration. Multiple investigators have successfully incorporated tetracycline hydrochloride and metronidazole benzoate into different polymeric solutions aiming to develop a material with therapeutic properties [9].
ii. Bioactive calcium phosphate incorporation: Co-electrospinning of hydroxyapatite and collagen with polymers has made possible the fabrication of membranes with improved mechanical properties. Studies on the membrane prepared by Liao et al. demonstrated that the addition of nano-carbonated hydroxyapatite improved both the biocompatibility and the osteoconductivity of the membrane [10]. Phipps et al (2011) fabricated a tri-component biocomposite fibrous electrospun membrane comprised of polycaprolactone, collagen and nanohydroxyapatite (50/30/20 weight ratio). The tri-component substrate exhibited a rapid spreading and significantly greater proliferation of cultured mesenchymal stem cells [7].
· Hydrogels: Hydrogels also offer great versatility in design and synthesis. They are sufficiently conductive to the migration of cells in to the bulk of the scaffolds and their biological properties such as bioactivity can be tuned and biomolecules (e.g., growth factors) can be added to guide, enhance or prevent cell adhesion and growth. Possibilities to enhance mechanical strength of hydrogels are in the generation of interpenetrating or nanocomposite hydrogel networks. Often a combination of different crosslinking mechanisms allows for generating mechanical strength, resilience and self-healing characteristics, all of which are suitable parameters for use as a periodontal regeneration membrane [7].
Cementum regeneration
A true and complete regeneration of cementum has remained an elusive goal in periodontal therapy. This is attributed to: i) reduced pool of available progenitor and stem cells; ii) cementoblast development, expression of osteocalcin and bone sialoprotein are required, while periodontal ligament (PDL) cells lack the expression of these proteins; iii) signalling molecules needed for recruiting cementoblasts and their differentiation (cemental derived growth factor) and for the attachment of new cementum to old cementum, cemental attachment proteins are required which are in low level in the wound healing environment.
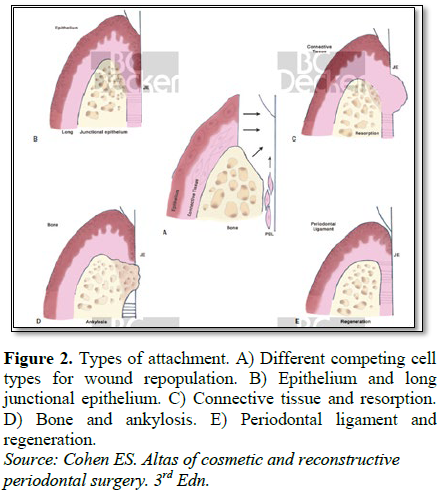
In spite of advances in the tissue engineering concept, we are only able to regenerate cellular cementum and not acellular cementum. Further, no single technique to regenerate cementum is successful and a combination of techniques is required. Reformation of acellular cementum during adult life becomes illusionary since the epithelial cells responsible for the formation of the hyaline layer are no longer present in the adult periodontium. In future by designing advanced technology to deliver novel genes and growth factors expressed by cementoblasts but absent in wound environment will assist in providing optimal conditions required for more predictable technique for cementum regeneration. Further, the extent of regeneration necessary to influence long-term tooth prognosis has not been established [11] (Figure 2).
Limited osteogenic potential of resident alveolar bone
Defects beyond certain size may not be completely restored by PDL cells. Aukhil [12] mentioned 2 factors responsible for this: i) slow rate of angiogenesis in PDL space consisting of avascular tissue; ii) premature differentiation of progenitor cells into formative cells result in delayed migration at the leading front. Osteogenic potential of the resident bone plays a role in periodontal regeneration [12]. Hence, higher alveolar bone level and narrow defect width will create better circumstances for complete regeneration.
Root surface alterations
Periodontitis renders the root surface toxic and unsuitable for the new connective tissue attachment necessary for periodontal regeneration, allowing down growth of junctional and pocket epithelium and inhibiting the regenerative progenitor cell attachment [13]. In the concept of reciprocal utilisation of biological mediators conducive for periodontal regeneration, root conditioning as a mode to attain periodontal regeneration was attempted. Despite the apparent sense in trying to improve the biological compatibility of the root surfaces using various conditioning agents, clinical results were disappointing [14]. Irrespective of the type of demineralizing agent used, it cannot be claimed that demineralization of the root surface per se is a regenerative procedure [5]. Despite the demineralization of the root surface by acids or chelating agents, the dentin itself is still mineralized and thus a real interconnection of the different fiber systems does not take place even though a new cementum layer is deposited on top of the cleaned, previously diseased root surface. According to some investigators, regenerative cementogenesis along established but formerly diseased and denatured root surfaces is not achieved in the true sense of the term [15]. Thus, root conditioning may have a positive effect on wound healing and be used as a component of, or a step within, various regenerative procedures.
SURGICAL TECHNIQUES
The conventional surgical techniques employed in regeneration under microscope have shown to be more traumatic causing tissue injury and thus impairs the periodontal regeneration. Flaps should be carefully designed and executed to ensure passive closure without tension on the wound margins. Realistically, true healing by primary intention is often difficult to achieve. However, primary wound closure is a fundamental surgical principle for GBR because it creates an environment that is undisturbed/unaltered by outside bacterial or mechanical insult. Passive closure of wound edges enables the wound to heal with less re-epithelialization, collagen formation and remodeling, wound contraction, and overall tissue remodeling. Techniques that have been advocated in an effort to gain tension-free primary wound closure include lateral incision technique, buccal rotational flap, coronally positioned palatal sliding flap, split palatal rotated flap and a palatal advanced flap [16]. Minimally invasive microscopic surgical techniques have been introduced and shown to be very promising [17]. Further, surgical flap techniques which causes least tissue loss and better flap adaptation should be adopted. E.g. simplified papilla preservation flap, modified papilla preservation flap etc. Thus, correct flap design, tension-free flap approximation, and postoperative care of the wound site are keys to achieving, and maintaining primary closure.
WOUND HEALING
Wound stabilization is a very critical factor in periodontal regeneration [18]. Care should be taken to maintain the integrity and stability of the clot during wound maturation and development. Wound integrity during the early healing phase rests primarily with that offered by suturing and the tooth-mucogingival flap interface is vulnerable to disruption by mechanical forces for a considerable time post-surgery. This knowledge underscores the importance of positioning and maintaining the mucogingival flaps protected from mechanical insult [19]. A thick blood clot seen during healing acts as an invitee for inflammatory cells for its resolution creating a dead space between the flap margin and tooth surface hampering regeneration. Therefore, it should be aimed to maintain a thin and stable blood clot by eliminating occlusal trauma, tooth mobility, giving nutritional supplements and enhancing wound healing by incorporating platelet concentrates. It has been postulated that near-final boundaries of the regenerated periodontal tissues should be established within 4 weeks, if not earlier. Strategies based on long-term availability of biological factors or slowly resorbing materials and devices, are unlikely to support clinically meaningful improvements and should thus be reconsidered. By thorough understanding with proper integration and controlling the factors which aid in wound healing would result in more predictable regenerative outcome [20].
REGENERATIVE MATERIALS
Bone grafts
The rationale behind the use of bone fillers is to take advantage of one or more of the following properties of such materials, namely osteoconduction, osteoinduction and osteogenesis, induced by transferred cells that are capable of differentiating into osteoblasts [21-23]. Not all three properties apply to every type of bone filler. The available evidence from human histological studies indicate that use of autogenous bone, demineralized freeze dried bone allograft and bovine-derived xenograft may result in periodontal regeneration. On the contrary, there is no evidence demonstrating predictable regeneration following use of alloplastic materials. However, the basic problem pertaining to all bone filler materials is that a biologic rationale for the regeneration of the periodontium is missing. Bone grafts or bone substitute materials do not possess the ability to regenerate lost connective tissue attachment. The osteoconductive, osteoinductive and/or osteogenic properties of such materials can at best support new bone formation [1]. Recently, newer drugs have been proposed which have shown promising results in terms of periodontal regeneration.
· Nanoparticles of hydroxyapatite have emerged as bioactive substances having positive osteogenic activity which have shown to facilitate the bioactivity of osteoblasts which promotes quicker bone regeneration [24].
· Supplementation with metal ions (zinc, strontium, magnesium)
Recently, metal ions of zinc, strontium and magnesium have been incorporated in hydroxyapatite (HA) particles to improve the therapeutic benefits of periodontal treatment. These elements invigorate bone growth and bone mineralization by initiation of osteogenesis of osteoblast cells aside from restraining osteoclastic resorption. Further, the calcium present in HA can be exchangeable for metal ions and presence of these elements in HA may cocreate bone cells, which take part in bone turnover and remodelling [24].
Addition of bisphosphonates to HA: Osteoporosis therapeutics has been dominated by anti-resorptive agents, mainly bisphosphonates (risedronate, alendronate, zoledronic acid, etc.) which prevent further bone loss in established osteoporosis but do not change the bone mass or replenish the already lost bone. Owing to their high affinity for HA, bisphosphonates after intravenous or oral administration are rapidly cleared from circulation and localize to the bone surface at the sites of active bone remodeling, especially in areas undergoing resorption by osteoclast. They have also been proposed to have osteostimulative properties in vivo and in vitro by an increase in the matrix formation [25]. The current evidence suggests that adjunctive bisphosphonate therapy appears to be effective in improving clinical outcomes of periodontitis in the short term. However, due to potential risk of osteoradionecrosis and short-term follow-up period of the studies, the clinical application of adjunctive bisphosphonate delivery in the management of periodontitis patients is debatable [26].
Porous titanium granules: The osteogenic properties of titanium have been studied in experimental studies and it has been shown that titanium stimulates activation of the complement system, surface binding of platelets, and platelet activation as reflected by increased levels of platelet-derived growth factor related to other tested materials [27].
Chitosan: Chitosan, a deacetylated derivative of chitin, is another biomaterial used for guided tissue regeneration that is biodegradable. Its property of bacteriostasis may reduce the bacterial contamination and benefit periodontal tissue regeneration. Chitosan gel has demonstrated promising effects on periodontal regeneration in human patients. Furthermore, chitosan has high osteoinductivity and osteo-integrability that make it a good candidate for bone regeneration [2,28].
Enamel matrix proteins
An important advancement in periodontal regeneration was the discovery of enamel matrix proteins (EMP), produced by Hertwig’s epithelial sheath. These proteins were shown to play an important role in cementogenesis, as well as in the development of the periodontal attachment apparatus. This observation led to the development and utilization of the biologically active agent “enamel matrix derivative” (Emdogain; Straumann AG, Basel, Switzerland) as a local adjunct to periodontal surgery for stimulating regeneration of periodontal tissues [5]. Emdogain is a purified acidic extract of developing embryonal enamel derived from six-month-old piglets. Its purpose is to act as a tissue-healing modulator that would mimic the events that occur during root development and to help stimulate periodontal regeneration [29]. It can act as a multipurpose growth factor capable of stimulating the proliferation of mesenchymal cells while inhibiting the cell division of epithelial cells [30]. However, the results cannot always be consistent. There are several reasons for this, including the use of: (i) different types of EMPs; (ii) different concentrations of EMPs; (iii) different observation periods; (iv) different cell types; (v) different states of cell differentiation; (v) different experimental in vitro systems or conditions; and (vi) different local in vivo environments. Nevertheless, there is a large body of information available that provides a biological rationale for the use of EMPs for periodontal regeneration [31]. This product is very well suited to sites where flap reflection is minimal since it’s positioning does not require any flap extension and the improved stability provided by minimally invasive surgery to the wound seems to favor the expression of its activity [32].
TISSUE ENGINEERING
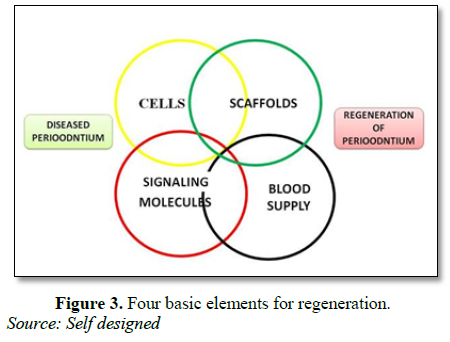
The use of biologic factors as biomimetic molecules to enhance regenerative response is based on the rationale of the local enrichment of natural biomolecules that may be deficient in chronic periodontal wound sites [33]. Current candidate and commercially available biologics (matrix/growth/differentiation factors), intended for periodontal indications, appear safe, with minimal (if any) adverse events associated with their clinical application. These molecules include: extracellular matrix proteins, cell-attachment factors, mediators of cell metabolism and activity and growth/differentiation factors. The most promising growth factors appear to be Bone Morphogenetic Protein (BMPs) [2,7]. Critical issues include: (i) the complexity of the periodontium consisting four different tissues; (ii) use of very high doses of BMPs; (iii) the ideal carrier has still not been found; (iv) enormous costs associated with recombinant human BMPs in relation to relatively small and non-life-threatening periodontal defects for which other treatment options exist [1]. The use of platelet rich fibrin (PRF) as a tissue engineering scaffold has been investigated by many researchers for the past few years. In a study by Gassling et al. [34] reported that PRF appears to be superior to collagen as a scaffold for human periosteal cell proliferation and PRF membranes can be used for in vitro cultivation of periosteal cells for bone-tissue engineering. In spite of the lack of scientifically proven clinical benefits, PRF is considered as a healing biomaterial and is commonly used in implant and plastic periodontal surgical procedures to enhance bone regeneration and soft tissue wound healing [34] (Figure 3).
GENE THERAPY
Despite the emerging evidence that local application of growth factors may encourage periodontal regeneration, a number of issues remain which limit their efficacy. These include containment of the factor at the local site, limited controlled release of the bioactive peptides, and inactivation of the growth factor via locally produced proteinases. As a result, more refined techniques have been explored to improve growth factor delivery and release for periodontal regeneration. One such method is gene transfer, whereby genes for regeneration-promoting growth factors using plasmid and adenovirus gene delivery methods are used [5]. However, there are certain limitations in the use of gene therapy which include: i) short-lived nature of gene therapy; ii) immune response of the patient; iii) problems with viral vectors like patient toxicity, immune and inflammatory responses, and gene control and targeting issues; iv) limitation of sufficient quantity of the engineered gene that can be delivered; v) extreme cost; vi) ethical restrictions [35]. Consequently, there is still a considerable amount of further work required before such an approach becomes a clinical reality [5].
STEM-CELL THERAPY
The discovery of periodontal ligament stem cells (e.g. Hertwig’s epithelial root sheath cells) has opened a new vista for periodontal regeneration. More recently, the use of periodontal ligament stem cells for tissue engineering approaches to facilitate periodontal regeneration has emerged. To date most of the studies have been restricted to experimental animals, with only one report involving transplantation of periodontal ligament stem cells into human periodontal defects being published [36,37]. A significant issue with these studies is that surgically created periodontal defects are very different from defects arising from periodontitis and thus any extrapolation of findings for stem cell regeneration in surgically created defects and what may happen in periodontitis needs to be made with caution [5]. Clinical challenges in stem cell-based periodontal therapy relate to immune rejection after administration, oncogenic properties of stem cells and functional integration of transplanted tissues into the host. A potential solution to this problem lies in the use of autologous stem cells (from cell/tissue banks) to overcome immune rejection. Further, the technical challenges in stem cell therapy are associated with cell manipulations, scaffold materials and delivery systems. The ideal matrix scaffold should mimic native extracellular matrix, support cell attachment, allow controlled release of bioactive factors, be conducive to tissue ingrowth and facilitate laboratory handling [38]. This led to the application of using cell sheet technology in conjunction with regenerative principles to deliver the regenerative potential of the periodontal ligament stem cells to the appropriate location [39].
RISK FACTORS
Bacterial accumulation
Bacterial accumulations following regenerative procedures would result in a reduced net formation of connective tissue and bone [35]. Mombelli et al. [32] evaluated the microbial contamination of expanded polytetrafluoroethylene membranes 6 weeks after surgical placement in ten patients. Porphyromonas gingivalis was found at high levels in one patient and Prevotella intermedia were found in six of the nine patients. The authors concluded that GTR membranes appeared to harbor periodontal pathogens on a frequent basis. Therefore, it is extremely important to control and/or reduce the bacterial contamination of the periodontal defect in order to enhance periodontal regeneration [32]. Bottino et al. [8] incorporated 25 weight % of metronidazole benzoate to functionally graded layer of GTR membrane interfacing with the epithelial tissue and showed reduction in bacterial growth and biofilm control.
Inadequate plaque control
Poor plaque control and residual periodontal infection have been associated with less favorable clinical outcomes following regenerative therapy.
Patients with inadequate supportive periodontal treatment after successful regenerative therapy demonstrate 50-fold increase in risk of probing attachment loss compared with those having regular recall visits [40].
Environmental factors
Smoking is a strong risk factor for adverse outcomes of regenerative therapy. The mechanisms by which smoking influences the clinical outcomes of regenerative procedures are purely speculative, but many of the known biological effects of smoking would certainly be expected to adversely influence both periodontitis and regeneration which include direct and indirect effects on vasculature, impairment of neutrophil function, interference with collagen biosynthesis and maintenance and altered immune-inflammatory responses [41].
Genetic factors
Gene mutation and polymorphism have shown to influence the outcome of regeneration and it is not possible to control the genetic influence, e.g. Interleukin-1ß. As only a proportion of the genetically predisposed and/or pathogen‐exposed individuals develop a disease, simple genetic explanations for individual susceptibility to chronic inflammatory diseases such as periodontitis have not been forthcoming [42].
Aging
The proliferative and migratory potential and the differentiation capacity of periodontal ligament stem cells decrease as age increases [43]. Aging negatively influences cell proliferation and mineral nodule formation. mRNA levels for type I and type III collagen are significantly lower in aged cells. Aging modulates important biological properties of periodontal ligament cells, diminishes the potential for mineral nodule formation and favors extracellular matrix degradation. [44]. Aging influences specific components of the wound healing process which is especially prominent in surgical wounds in which there is some early ischemia. Ischemic wounds in older animals have significantly lower numbers of mononuclear cells infiltrating the wounds and display a reduced strength, as compared to younger animals [45].
Systemic factors
Of all the systemic diseases that are relatively common, diabetes mellitus has emerged in recent years as the one with the strongest potential influence on periodontal diseases. Diabetes does not directly alter the plaque composition but is likely to alter the tissue response to the bacteria. One component of the increased risk may be an increased microbial challenge and the other component may be a delayed wound healing response that is most likely the result of poor control of glucose metabolism on the inflammatory process. Thus, the risk of failure in diabetics is likely to be related to the stability of long-term control of glucose metabolism. Improved metabolic control is currently the only practical approach to managing this risk factor. Stringent measures have to be taken to maintain adequate plaque control and to negate the negative influence of these factors. Further, patients who are non-compliant and have uncontrolled systemic conditions should not be taken up as they affect the predictable outcome of periodontal regeneration [41].
Role of occlusion
Occlusal forces that exceed physiological thresholds (normal forces in the molar and bicuspid region being around 120 to 150 N), whether a function of force, magnitude, duration or direction, do not initiate gingivitis or periodontitis but can result in bone remodelling and tooth mobility. Occlusal forces of sufficient magnitude to cause severe or increasing tooth hypermobility may accelerate attachment loss in existing, plaque-induced periodontitis and, in addition, may interfere with repair or regeneration of connective tissue attachment lost to periodontitis [41]. The diagnosis of trauma from occlusion is based on a functional analysis of occlusal relations, the investing and supporting structures of the teeth, the muscles of mastication and the teeth. The analysis combines both a clinical and radiographic examination. The clinician must recognise progressing tooth mobility due to trauma, teeth under premature centric occlusal contacts and/or under traumatic excursional interferences. Such occlusal discrepancies should be adjusted to minimize trauma and thus tooth mobility prior to regenerative therapy [45]. Burgett et al. [46] found that teeth which received occlusal adjustments prior to surgical treatment resulted in a greater attachment level gain when compared to non-adjusted controls.
METHODS OF ASSESSING PERIODONTAL REGENERATION
The primary methods used for evaluation include histology, direct measurement of bone, periodontal probing and radiographic analysis. The correlation between clinical attachment level measurements and histology as it relates to regeneration is not as clear as the subtraction radiography and histology data. Digital subtraction radiography has been validated to quantitative bone loss or gains in terms of three-dimensional volume or bone mass destroyed or regenerated. For the long term, the power of radiographic image processing for regeneration therapy lies not only in the ability to detect a small lesion but in the ability to detect and size that lesion in terms of length, area, volume and mass of bone gain [47]. Assessment is done after an interval of 6 months which leads to disruption of the healing process. Additionally, assessment is subject to errors which could give false positive results. While re-entry surgery or radiographs demonstrate impressive volume gains, the actual ratio of filler material to new bone cannot be determined using these methods [1]. The advent of cone-beam computed tomography (CBCT) has become one of the most useful advances in periodontology. CBCT has the potential to streamline clinical workflow; enhance the periodontist’s skills in diagnosis and complex treatment planning; hone surgical anatomic expertise; and improve outcomes in specific implant and periodontal cases. However, minimally invasive therapies and evaluation of regenerative procedure outcomes are two areas of potential benefit where further research was indicated [48]. Newer technologies that are non-invasive provide real time assessment and are more objective with no bias in measurements should be developed for a true and holistic assessment.
CONCLUSION
Our perspective on the current evidence is that regenerative periodontal therapies to date can only restore a fraction of the original tissue volume in extent. Thus, complete periodontal restoration may still be regarded as an illusion. Finally, it should still be borne in mind that the structural and interactive complexity of periodontal tissues is probably one of the reasons why it is so difficult to regenerate the periodontium. New knowledge about the etiology and pathogenesis of periodontitis, the relationship of disease to systemic problems, and advances in genetics, molecular biology, cell biology and biomaterials, have opened the door for new regenerative techniques based upon the tissue engineering approach. There is not going to be one magic solution that can be used to treat all periodontal patients, but rather a combination of different approaches that can be adjusted to fit the specific need of individual patients.
1. Bosshardt DD, Sculean A (2009) Does periodontal tissue regeneration really work? Periodontol 2000 51: 208-219.
2. Khajuria DK, Zahra SF, Razdan R (2018) Effect of locally administered novel biodegradable chitosan based risedronate/zinc-hydroxyapatite intra-pocket dental film on alveolar bone density in rat model of periodontitis. J Biomater Sci Polym Ed 29: 74-91.
3. Melcher AH (1976) On the repair potential of periodontal tissues. J Periodontol 47: 256-260.
4. Caton JG, Greenstein G (1993) Factors related to periodontal regeneration. Periodontol 2000 1: 9-15.
5. Bartold PM (2015) Group C. Initiator paper. Periodontal regeneration - Fact or fiction. J Int Acad Periodontol 17: 37-49.
6. Bröseler F, Tietmann C, Hinz AK, Jepsen S, Čebatariūnienė A, et al. (2005) Position paper: Periodontal regeneration. J Periodontol 76: 1601-1622.
7. Bottino MC, Thomas V, Schmidt G, Vohra YK, Chu TM, et al. (2012) Recent advances in the development of GTR/GBR membranes for periodontal regeneration - A materials perspective. Dent Mater 28: 703-721.
8. Bottino MC, Thomas V, Janowski GM (2011) A novel spatially designed and functionally graded electrospun membrane for periodontal regeneration. Acta Biomater 7: 216-224.
9. Zamani M, Morshed M, Varshosaz J, Jannesari M (2010) Controlled release of metronidazole benzoate from poly epsilon-caprolactone electrospun nanofibers for periodontal diseases. Eur J Pharm Biopharm 75: 179-185.
10. Liao S, Wang W, Uo M, Ohkawa S, Akasaka T, et al. (2005) A three-layered nano-carbonated hydroxyapatite/collagen/PLGA composite membrane for guided tissue regeneration. Biomater 26: 7564-7571.
11. Garrett S (1996) Periodontal regeneration around natural teeth. Ann Periodontol 1: 621-666.
12. Polimeni G, Albandar JM, Wikesjo¨UME (2004) Prognostic factors for alveolar regeneration: Osteogenic potential of resident bone. J Clin Periodontol 31: 840-844.
13. Polson AM, Caton J (1982) Factors influencing periodontal repair and regeneration. J Periodontol 53: 617-625.
14. Mariotti A (2003) Efficacy of chemical root surface modifiers in the treatment of periodontal disease. A systematic review. J Periodontol 8: 205-226.
15. Meyle J (2014) Group C reactor paper periodontal regeneration - Fact or fiction? J Int Acad Periodontol 11: 50-53.
16. Wang HL, Boyapati L (2006) “PASS” principles for predictable bone regeneration. Implant Dent 15: 8-17.
17. Cortellini P, Tonetti MS (2007) A minimally invasive surgical technique with an enamel matrix derivative in the regenerative treatment of intra‐bony defects: A novel approach to limit morbidity. J Clin Periodontol 34: 87-93.
18. Wikesjo UME, Nilvkus R (1990) Periodontal repair in dogs: Effect of wound stabilization on healing. J Periodontol 61: 719-724.
19. Wikesjö UM, Selvig KA (1999) Periodontal wound healing and regeneration. Periodontol 2000 19: 21-39.
20. Susin C, Wikesjö UM (2013) Regenerative periodontal therapy: 30 years of lessons learned and unlearned. Periodontol 2000 62: 232-242.
21. Bosshardt DD, Hjørting-Hansen E, Buser D (2009) The fate of the autogenous bone graft. Forum Implantologicum 5: 4-11.
22. Bosshardt DD, Schenk RK (2009) Bone regeneration: Biologic basis. In: Buser D, editor. 20 Years of Guided Bone Regeneration in Implant Dentistry. 2nd Edn. Chicago, IL: Quintessenz, pp: 15-45.
23. Jensen SS, Bosshardt DD, Buser D (2009) Bone grafts and bone substitute materials for GBR procedures. In: Buser D, editor. 20 Years of Guided Bone Regeneration in Implant Dentistry. 2nd Edn. Chicago, IL: Quintessenz, pp: 71-96.
24. Khajuria DK, Vasireddi R, Trebbin M, Karasik D, Razdan R (2017) Novel therapeutic intervention for osteoporosis prepared with strontium hydroxyapatite and zoledronic acid: In vitro and pharmacodynamic evaluation. Mater Sci Eng C Mater Biol Appl 71: 698-708.
25. Khajuria DK, Razdan R, Mahapatra DR (2015) Development, in vitro and in vivo characterization of zoledronic acid functionalized hydroxyapatite nanoparticle based formulation for treatment of osteoporosis in animal model. Eur J Pharm Sci 66: 173-183.
26. Akram Z, Abduljabbar T, Kellesarian SV, Hassan A, Ibrahim M, et al. (2017) Efficacy of bisphosphonate as an adjunct to nonsurgical periodontal therapy in the management of periodontal disease: A systematic review. Br J Clin Pharmacol 83: 444-454.
27. Thor A, Rasmusson L, Wennerberg A, Thomsen P, Hirsch JM, et al. (2007) The role of whole blood in thrombin generation in contact with various titanium surfaces. Biomater 28: 966-974.
28. Boynueğri D, Özcan G, Şenel S, Uç D, Uraz A, et al. (2009) Clinical and radiographic evaluations of chitosan gel in periodontal intraosseous defects: A pilot study. J Biomed Mater Res Part B 90: 461-466.
29. Venezia E, Goldstein M, Boyan BD, Schwartz Z (2004) The use of enamel matrix derivative in the treatment of periodontal defects: A literature review and meta-analysis. Crit Rev Oral Biol Med 15: 382-402.
30. Zeichner-David M (2006) Regeneration of periodontal tissues: Cementogenesis revisited. Periodontol 2000 41: 196-217.
31. Bosshardt DD (2008) Biological mediators and periodontal regeneration: A review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol 35: 87-105.
32. Cortellini P, Pini-Prato G, Nieri M, Tonetti MS (2009a) Minimally invasive surgical technique and enamel matrix derivative (EMD) in intrabony defects: Factors associated with healing outcomes. Int J Periodontics Restorative Dent 29: 256-265.
33. Giannobile WV (2014) Treatment of periodontitis: Destroyed periodontal tissues can be regenerated under certain conditions. J Periodontol 85: 1151-1154.
34. Gassling V, Douglas T, Warnke PH, Açil Y, Wiltfang J (2010) Platelet‐rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implants Res 21: 543-549.
35. Karthikeyan BV, Pradeep AR (2006) Gene therapy in periodontics: A review and future implications. J Contemp Dent Pract 7: 83-91.
36. Feng F, Akiyama K, Liu Y, Yamaza T, Wang TM, et al. (2010) Utility of PDL progenitors for in vivo tissue regeneration: A report of 3 cases. Oral Dis 16: 20-28.
37. Hynes K, Menicanin D, Gronthos S, Bartold PM (2012) Clinical utility of stem cells for periodontal regeneration. Periodontol 2000 59: 203-227.
38. Lin NH, Gronthos S, Bartold PM (2009) Stem cells and future periodontal regeneration. Periodontol 2000 51: 239-251.
39. Washio K, Iwata T, Mizutani M, Ando T, Yamato M, et al. (2010) Assessment of cell sheets derived from human periodontal ligament cells: A pre-clinical study. Cell Tissue Res 341: 397-404.
40. Cortellini P, Pini‐Prato G, Tonetti M (1994) Periodontal regeneration of infrabony defects (V). Effect of oral hygiene on long‐term stability. J Clin Periodontol 21: 606-610.
41. Kornman KS, Robertson PB (2000) Fundamental principles affecting the outcomes of therapy for osseous lesions. Periodontol 2000 22: 22-43.
42. Renz H, Von Mutius E, Brandtzaeg P, Cookson WO, Autenrieth IB, et al. (2011) Gene-environment interactions in chronic inflammatory disease. Nat Immunol 12: 273-277.
43. Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, et al. (2012) The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomater 33: 6974-6986.
44. Benatti BB, Silvério KG, Casati MZ, Sallum EA, Nociti Jr FH (2008) Influence of aging on biological properties of periodontal ligament cells. Connect Tissue Res 49: 401-408.
45. Trejo PM, Weltman RL (2004) Favourable periodontal regenerative outcomes from teeth with presurgical mobility: A retrospective study. J Periodontal 75: 1532-1538.
46. Burgett FG, Ramfjord SP, Nissel RR, Morrison EC, Charveneau TD et al. (1992) A randomised trial of occlusal adjustments in the treatment of periodontitis patients. J Clin Periodontol 19: 381-387.
47. Reddy MS, Jeffcoat MK (1999) Methods of assessing periodontal regeneration. Periodontol 2000 19: 87-103.
48. McAllister BS, Eshraghi VT (2017) Commentary: Cone-beam computed tomography: An essential technology for management of complex periodontal and implant cases. J Periodontol 88: 937-938.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)