586
Views & Citations10
Likes & Shares
Coronary slow flow (CSF) is a vascular phenomenon which is detected by
angiography images, created using a contrast dye, that are characterized by
delayed distal vessel opacification without any significant epicardial coronary
artery stenosis. This phenomenon, in clinical practice, is observed with a
respectively high incidence, a rate of 7% in patients who have diagnostic
coronary angiography [1]. CSF is seen in
higher rates with patients who are young male smokers. Patients who have CSF
might have a variety of symptoms from being asymptomatic to typical angina or
unstable angina with a diagnosis of acute coronary syndrome [2].
Since this phenomenon was first identified, multiple hypotheses have
been proposed to enlighten the pathophysiology of CSF, including small vessel
disease, microvascular vasomotor dysfunction, diffuse atherosclerosis and
endothelial dysfunction. But these pathophysiological mechanisms remained as
hypotheses and the exact mechanism for this angiographic phenomenon had never
been fully understood. Mosseri et al. [3] hypothesized that local small vessel
dysfunction was the reason behind CSF and this hypothesis was supported by myocardial
biopsies in research which revealed a loss of luminal size due to thickening of
vessel walls during coronary microcirculation. But further research, in 1996,
by Beltrame et al. [4] indicated a decreased response to endothelial stimuli in
CSF patients. After these studies, intravascular ultrasound (IVUS) was used to
observe the vessel thickening. Researchers showed that CSF patients had diffuse
intimal thickening together with calcification which did not cause any luminal
irregularities in the coronary angiography. Similarly in 2004, Pekdemir et al.
[5] demonstrated that CSF patients had extended and widespread calcification in
the epicardial coronary arteries and suggested that these calcifications may be
a preliminary sign or cause of atherosclerotic disease in the coronary
arteries; furthermore, CSF could be a form of early detection for
atherosclerosis, a condition which affects the microvascular circulation [5]. Another finding revealed the relationship
between ectasia in the coronary arteries and slow flow [6]. It is known that the velocity of fluids in
pipes can be altered when the pipe is suddenly enlarged or curved. Accordingly,
abrupt changes in the vessels, like ectasia, can create a flow that might be
slower compared to a vessel with ideal conditions. Based on the research,
pathophysiology behind the CSF might be suggested not only as a structural
problem but also as a microcirculatory dysfunction in the coronary arteries.
On the other hand, the assessment of flow-mediated dilatation (FMD) of
the brachial artery has been widely used to investigate the endothelial
function of the arteries. One of the studies showed that there was a
concomitant relationship between CSF and FMD of the brachial artery. Patients
who had CSF also showed reduced endothelial-dependent FMD of the brachial
artery in an ultrasound [7]. Another
finding indicated the concentrations of nitric oxide (NO) and endothelin-1
(ET-1) were lower in the CSF patients [8].
Kurtoglu et al. [9] investigated the effect of dipyridamole treatment which
showed beneficial progress in restoring the flow in these patients. In another
study, Tanriverdi et al. [10] showed impairment of endothelial function due to
homocysteine induced oxidative stress in CSF patients. These findings suggest
that CSF might be associated not only with local disease but with systemically
affected endothelial dysfunction.
Both this systematical involvement and the microcirculation abnormality of coronary arteries may be associated with impaired choroidal microcirculation. It has been shown in animal models that atherosclerotic changes occurred in choroidal arteries [11]. Studies showed that subfoveal choroidal thickness (SFCT) decreased in the patients who had retinitis pigmentosa due to a microcirculation abnormality [12,13]. These findings revealed the obvious relationship between vascular beds. In 2014, Ahmad et al. [14] showed that patients with coronary artery diseases had a thinner macular choroid than controls. In 2014, Altinkaynak et al. [15] showed that patients with congestive heart failure presented lower SFCT compared to age- and gender-matched controls. Another study indicated a close relationship between CSF and SFCT; additionally, the study demonstrated improvement of SFCT with the treatment of statin therapy [16].
Patients with CSF may also present with other clinical features. Yilmaz
et al. [17] studied the clinical and laboratory relationships of CSF patients
and found a close relationship between CSF and the following clinical problems:
insulin resistance, impaired glucose tolerance, metabolic syndrome with the
presence of higher total cholesterol, low-density lipoprotein cholesterol,
fasting glucose and body mass index. Therefore, anti-inflammatory statin
therapies are studied for patients who have CSF and mentioned clinical
problems.
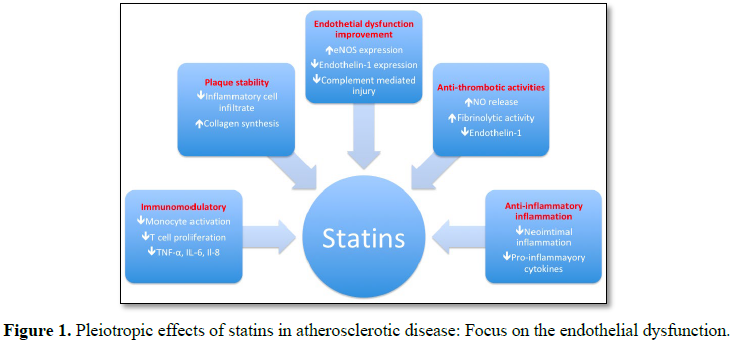
Statins work in a variety of ways to effect CSF patients (Figure 1). Statins can effectively
lower cholesterol levels by inhibiting endogenous cholesterol synthesis. This
lowering effect might restore the endothelial function; however, trials showed
that endothelial function was restored in patients before the levels of lipids
were lowered, suggesting the cholesterol-independent effect of statins [18]. Taken together, endothelium dependent
vasodilation was triggered by statin therapy which was associated with lowering
cholesterol; statins also reduced the endothelin-1 release in endothelial cells
[19,20]. Another effect of statin treatment was the
modulation of the inflammatory process in the coronary arteries. Commonly,
statin treatment significantly lowered the high-sensitive C-reactive protein
(CRP) [21]. A statin lowered not only high-sensitive
CRP, but it also decreased the interleukin-6 (cultured mononuclear cell) levels
as well as inflammatory cytokine levels in in
vitro studies of human cells [22,23].
1. Mangieri
E, Macchiarelli G, Ciavolella M, Barilla F, Avella A, et al. (1996) Slow
coronary flow: Clinical and histopathological features in patients with
otherwise normal epicardial coronary arteries. Cathet Cardiovasc Diagn 37:
375-381.
2. Beltrame
JF, Limaye SB, Horowitz JD (2002) The coronary slow flow phenomenon - A new
coronary microvascular disorder. Cardiology 97: 197-202.
3. Mosseri
M, Yarom R, Gotsman MS, Hasin Y (1986) Histologic evidence for small vessel
coronary artery disease in patients with angina pectoris and patent large
coronary arteries. Circulation 74: 964-972.
4. Beltrame
JF, Limaye SB, Wuttke RD, Horowitz JD (2003) Coronary hemodynamic and metabolic
studies of the coronary slow flow phenomenon. Am Heart J 146: 84-90.
5. Pekdemir
H, Polat G, Cin VG, Camsari A, Cicek D, et al. (2004) Elevated plasma
endothelin-1 levels in coronary sinus during rapid right atrial pacing in
patients with slow coronary flow. Int J Cardiol 97: 35-41.
6. Senen
K, Yetkin E, Turhan H, Atak R, Sivri N, et al. (2004) Increased thrombolysis in
myocardial infarction frame counts in patients with isolated coronary artery
ectasia. Heart Vessels 19: 23-26.
7. Sezgin
AT, Sigirci A, Barutcu , I, Topal E, Sezgin N, et al. (2003) Vascular
endothelial function in patients with slow coronary flow. Coron Artery Dis 14:
155-161.
8. Camsarl
A, Pekdemir H, Cicek D, Polat G, Akkus MN, et al. (2003) Endothelin-1 and
nitric oxide concentrations and their response to exercise in patients with
slow coronary flow. Circ J 67: 1022-1028.
9. Kurtoglu
N, Akcay A, Dindar I (2001) Usefulness of oral dipyridamole therapy for
angiographic slow coronary artery flow. Am J Cardiol 87: 777-779.
10. Tanriverdi
H, Evrengul H, Enli Y, Kuru O, Seleci D, et al. (2007) Effect of
homocysteine-induced oxidative stress on endothelial function in coronary
slow-flow. Cardiology 107: 313-320.
11. Salazar
JJ, Ramírez AI, de Hoz R, Rojas B, Ruiz E, et al. (2007) Alterations in the
choroid in hypercholesterolemic rabbits: Reversibility after normalization of
cholesterol levels. Exp Eye Res 8: 412-422.
12. Balmforth
C, van Bragt JJ, Ruijs T, Cameron JR, Kimmitt R, et al. (2016) Chorioretinal
thinning in chronic kidney disease links to inflammation and endothelial
dysfunction. JCI Insight 1: 1-13.
13. Kim
H, Lee SC, Kwon KY, Lee CS (2016) Subfoveal choroidal thickness as a predictor
of treatment response to anti-vascular endothelial growth factor therapy for
polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 254:
1497-1503.
14. Ahmad
M, Kaszubski PA, Cobbs L, Reynolds H, Smith RT (2017) Choroidal thickness in
patients with coronary artery disease. PLoS One 12: 1-12.
15. Altinkaynak
H, Kara N, Sayın N, Güneş H, Avşar S, et al. (2014) Subfoveal choroidal
thickness in patients with chronic heart failure analyzed by spectral-domain
optical coherence tomography. Curr Eye Res 39: 1123-1128.
16. Kanar
BG, Kanar HS (2018) Relationship between angiographic coronary slow flow
phenomenon and subfoveal choroidal thickness: What is the effect of
atorvastatin therapy? Eur Exp Biol 8: 9.
17. Yilmaz
H, Demir I, Uyar Z (2008) Clinical and coronary angiographic characteristics of
patients with coronary slow flow. Acta Cardiol 63: 579-584.
18. Wassmann
S, Faul A, Hennen B, Scheller B, Bohm M, et al. (2003) Rapid effect of
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibition on coronary
endothelial function. Circ Res 93: 98-103.
19. Stroes
ES, Koomans HA, de Bruin TW, Rabelink TJ (1995) Vascular function in the
forearm of hypercholesterolemic patients off and on lipid-lowering medication.
Lancet 346: 467-471.
20. Hernandez-Perera
O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, et al.
(1998) Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors,
atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial
nitric oxide synthase in vascular endothelial cells. J Clin Invest 101:
2711-2719.
21. Plenge
JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, et al. (2002) Simvastatin
lowers C-reactive protein within 14 days: An effect independent of low-density lipoprotein
cholesterol reduction. Circulation 106: 1447-1452.
22. Jialal
I, Stein D, Balis D, Grundy SM, Adams-Huet B, et al. (2001) Effect of
hydroxymethyl glutaryl coenzyme A reductase inhibitor therapy on high sensitive
C-reactive protein. Circulation 103: 1933-1935.
23. Weber
C, Erl W, Weber KS, Weber PC (1997) HMG-CoA reductase inhibitors decrease CD11b
expression and CD11b-dependent adhesion of monocytes to endothelium and reduce
increased adhesiveness of monocytes isolated from patients with hypercholesterolemia.
J Am Coll Cardiol 30: 1212-1217.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Food and Nutrition-Current Research (ISSN:2638-1095)