907
Views & Citations10
Likes & Shares
Background: The increased
incidence of sexually transmitted infections (STIs) in Brazil represents a
significant Public Health issue. The natural latex male condom is a resource
available to both men and a woman that meets the dual protective function. In
Brazil, male condoms need to be certified. The certification process evaluates
the manufacturing and quality of the final product in detail. However, the post
market surveillance is not part of the normal practice. Public health officials
have warned of the emergence of new Sexually Transmitted Infections caused by
four bacteria and superbugs.
Methods: Two brands of male
condoms, one purchased in the Brazilian trade (Sample A) and the other
distributed by the Brazilian government program (Sample B) were evaluated. The
quality control of the male condom involves the important insufflation test,
which evaluates the resistance determining the volumetric capacity and the
rupture pressure. The microbiological assay was performed as a complement. For
the inflation test, we tested 200 units per brand, according to the criteria
established in the Resolution of the National Agency of Sanitary Surveillance
nº. RDC 62/2008 that allows up to seven nonconforming units. Microbiological
analyzes were tested following the Standard Operational Procedure (SOP) of the
National Institute of Health Quality Control (INCQS), Oswaldo Cruz Foundation,
Rio de Janeiro, Brazil, number 65.3210.008 rev. 15 according to the guidelines
established by the Brazilian Pharmacopoeia, 5th Edn, 2010.
Results and
conclusion: Both brands met the criteria established in the Resolution of the
National Sanitary Surveillance Agency no. RDC 62/2008 for the inflation test,
which allows up to seven nonconforming units. However, the microbiological
tests pointed out the presence of pathogens. We conclude that, in addition to
certification, there is a need to monitor this product in view of the sanitary
risk observed.
Keywords: Male condoms, Public health, Sanitary risk,
Sanitary surveillance
INTRODUCTION
Background of the study
The incidence of STIs had increased in Brazil and now represents a
significant Public Health issue. This increase is predominantly due to low
socioeconomic status, poor cultural conditions and lack of adequate sex
education, particularly among young individuals. Today, STIs are among the most
common diseases worldwide [1].
Globally, the health impact of STIs had led to allocation of huge funds
for treatment of young individuals in a range of countries. The World Health
Organization (WHO) estimated the rise in incidence of curable STIs in Brazil
from 10 million to 12 million annually, covering the age group of 15-49 years
[2]. In Brazil, the use of condoms remains an important prevention policy, the
Ministry of Health, in response to the increase in cases of STIs and
considering the potential consequences evaluated inconsistencies in the quality
of condoms and the population disinterest in use of condoms. To remedy this,
post exposure prophylaxis concomitant has been introduced for the treatment of
all high-risk individuals following sexual intercourse (vaginal, anal and oral)
since October 2010. This involves the administration of medications up to 72 h,
after sexual intercourse, if condoms were not used or failed during intercourse
[3].
The male condom serves a dual function by
protecting against both pregnancy and STIs [4]. It does not have any side
effects except to individuals who are allergic to latex.
New diseases emerge all the time and sexually transmitted infections
are no exception. Gonorrhea, chlamydia and syphilis are still the most common
STIs, but public health officials are increasingly warning of the prevalence of
four bacteria and superbugs [5].
Neisseria meningitidis can cause invasive meningitis, a potentially
deadly infection of the brain and spinal cord's protective membranes, more
commonly; it's gaining a reputation as a cause of urogenital infections.
Roughly 5-10% of adults carry N.
meningitidis in the back of the nose and throat. Studies suggest they can
potentially transmit the bacteria to partners through oral sex, deep kissing or
other kinds of close contact that transmit infected droplets. Researchers are
not yet sure which of these transmission routes have caused outbreaks of
invasive forms of the disease among gay and bisexual men in Europe, Canada and
the US. However, one study of urethritis caused by N. meningitidis in a separate group of men (all but one of whom
were heterosexual) suggested that they contracted it from receiving oral sex
[5].
A Mycoplasma genitalium
bacterium, one of the smallest bacteria known, is gaining an outsized
reputation as a worrisome STI. Identified in the 1980s, the bacterium today
infects an estimated 1-2% of people and is especially common in adolescents and
young adults [5].
M. genitalium infection, though often symptom-free, can
mimic chlamydia or gonorrhea with persistent irritation of the urethra and
cervix. Because it may trigger pelvic inflammatory disease in the female
reproductive system, it has been associated with infertility, miscarriage, premature
birth and even stillbirth. While condoms can help prevent infection,
researchers have sounded the alarm about M.
genitalium's growing resistance to treatment with the antibiotics
azithromycin and doxycycline [5].
Shigellosis (or Shigella
dysentery) is passed on by direct or indirect contact with human feces. The
infection causes severe stomach cramps and explosive bouts of blood- and
mucus-filled diarrhea, which helps perpetuate transmission of the bacteria.
Although the disease is most commonly associated with young children and
travellers in some low- and middle-income countries, researchers began
documenting cases of shigellosis in gay and bisexual men in the 1970s. S. flexneri, scientists believe,
essentially exploited a new niche for transmission through anal-oral sex and
has led to multiple STI outbreaks around the world since then [5].
Lymphogranuloma venereum (LGV) an STI caused by unusual strains of Chlamydia trachomatis, can cause an
“awful infection”, according to Christopher Schiessl, a doctor at the One
Medical clinic in San Francisco's Castro neighborhood. LGV may first produce a
temporary genital pimple, blister or ulcer and then invade the body's lymphatic
system. Rectal infection can mimic inflammatory bowel disease and lead to
chronic and severe colon and rectal abnormalities such as fistulas and
strictures [5].
Over the past decade, LGV has become increasingly common in Europe and
North America and has been associated with multiple disease outbreaks,
especially among gay and bisexual men. As with chlamydia, LGV can increase the
risk of contracting HIV [6].
The risk relationship, calculated on causes and effects, related to
probabilities, uncertainties, contrasts with several studies that point to the
existence of models, assumptions and techniques of subjective evaluation of
risk very different from the scientific models, for which the phases of the
processes are integrated, taking into account the social, environmental,
political and economic parameters [7].
For Sanitary Surveillance, risk is evaluated in processes, procedures,
environments, products and services of interest to health and the purpose of
their actions is to inhibit or minimize their effects on the health of the
population. With a focus on risk control, its practices integrate prevention of
diseases, protection and health promotion [8].
In Brazil, the quality of available male condoms is under the
jurisdiction of the National Health Surveillance Agency (ANVISA) of the
Ministry of Health of Brazil. The Resolution of the Collegiate Board of
Directors, RDC 62/2008, based on ISO 4074: 2002 establishes the Technical
Regulation for certification of this product [9,10].
Male condoms, like other products that may cause some kind of impact on
health, consumer safety or the environment, are compulsorily certified, that
is, they can be circulated for commercialization or distribution with the seal
of conformity, which certifies that that product meets the minimum requirements
of the quality established according, the standards that foresee its use.
However, the certification process, although it evaluates the production and
the product in detail at the end of the production, does not address the issue
of commercialization in several establishments such as pharmacies, drugstores
and supermarkets, considered typical sanitary surveillance actions[9].
The revisions of ISO 4074 in November 2015 highlight the importance of
microbiological evaluation in male condoms to prevent possible risks of
microbiological contamination. According to the revision of ISO 4074/2015, “It
is recommended that manufacturers establish procedures for periodically
monitoring microbial contamination (bio burden) as part of their quality
management system, including requirements for the absence of pathogens and
limits on the total viable count on specific finished condoms; methods of
determining “bio burden” levels for condoms [10,11]”.
The presence of poor quality condoms on the market represents a serious
challenge in the fight against STIs. The lack of quality of this product
affects the popular perception of the value of the condom, which, in turn, can
have a significant impact on Public Health. Therefore, they can harm not only
the health of users, but also the reputation of agencies or the national agency
that provides condoms [12]. The objective of this study was to evaluate, due to
the disclosed perspective of the emergence of new STDs [5], to show that we
should adopt a system of monitoring male condoms in a broad and active way in
an attempt to minimize sanitary risks, taking into account complementary essay
in the set of physical tests established in legislation [9,10].
METHODS
Two samples of condoms were collected for evaluation, following ISO
4074/2015. Samples of condoms were available on the market, through of consultation
to the ANVISA database and using a random draw to choose a brand from the
selected manufacturer. The other sample consisted of condoms from a free
distribution campaign conducted by the Brazilian Health Ministry. Sampling of
samples followed ISO 2859 [14].
Two brands of male condoms, anonymized by marking with letters, one
purchased in the Brazilian trade (sample A) and the other distributed by the
Brazilian government program (sample B). The analytical tests carried out on
the brands were the insufflation test and the microbiological.
Bursting volume and bursting pressure (insufflation test) were measured
in accordance to standards established by the Brazilian National Health
Oversight Agency Resolution no RDC 62/2008, using an eight-head automated
inflation system (Enersol™, Sydney, Australia). For this analysis, we used the
ISO 2859 single sampling plan, with a normal inspection regime, at inspection
level I (acceptable quality level (AQL)=1.5, less than 1.5% of units
defective). The number of units assessed was 200 per lot and the acceptance
criteria reject the lot if at least eight nonconforming units were identified.
The male condoms were placed in the eight-head automated inflation system the
flow of compressed air was set at 24-30 dm3 min-1, as
defined in the standards. For each condom, the bursting pressure (1 kPa=N force
applied uniformly over an area of 1 m2) and bursting volume (in dm3)
were logged via the EInflation3 software [13]. During the insufation test, the
condom is inflated like a balloon, stretching the latex film until its rupture,
thereby indicating its maximum resistance. The inflation system is accompanied
by software that logs the pressure and volume at bursting. The compressed air
that supplies the system is generated by a dry, oil-free air compressor [9,14].
Microbiological analyzes consist of quality control of non-sterile
drugs, cosmetics, health articles and supplies, treated water for dialysis and
polyelectrolyte concentrate for dialysis, as well as raw materials used in its
manufacture. The tests followed the Standardized Operational Procedure (SOP) of
the National Institute of Quality Control in Health (INCQS), Oswaldo Cruz
Foundation, Rio de Janeiro, Brazil, number 65.3210.008 rev. 15 according to the
guidelines established by the Brazilian Pharmacopoeia, 5th edition of 2010,
aiming at proving the existence of microorganisms such as Enterobacter, Staphylococus aureus and Pseudomonas aeruginosa specified in the Resolution
[15].
RESULTS
For physical tests, Table 1 presents AQL, amount of sample test and the criteria
established in accordance with RDC 62/2008.
For all regulations the overflow requirement
is linked to the width of the condom, measured at (75 ± 5) from the closed end
of the condom. The overflow volume should not be less than 16.0 dm3,
for condoms with nominal width less than 50.0 mm; 18.0 dm3, for
condoms with nominal width greater than 50.0 mm and less than 56.0 mm; 22.0 dm3,
for condoms greater than 56.0 mm and less than 65.0 mm; 28.0 dm3,
for condoms greater than or equal to 65.0 mm and less than or equal to 75.0 mm.
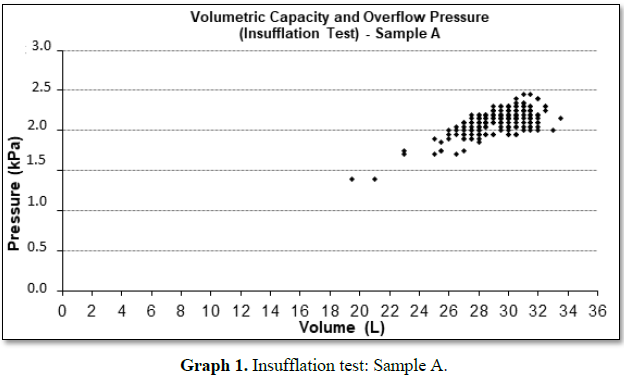
Graphs 1 and 2 represent the insufflation tests performed in
samples A and B, respectively. The variability for pressure and burst volume
shows that the units are not uniform. However, the distribution stretches to
the left, both for pressure and for volume, characterizing a non-normal
distribution contaminated by outliers, but within the conformity area, except
for sample B that presented unit of pressure and volume below the established
values.
By the characteristics
of the graphs, we noticed variability in the samples studied. This can be
explained by the fact that it is a mass produced product, influenced by the
properties of the latex film, the type of storage of the product to its use
among others, which highlights the importance of post-market monitoring beyond
certification (Table 2).
Microbiological analysis
The results for the
microbiological tests of both samples (A and B) indicated the presence of
pathogenic microorganisms. Table 3 presents the microbiological test
results for samples A and B, respectively.
The bacteria found are among the dominant etiological agents responsible
for more than 85% of cases of urinary tract infection, including Gram-negative
bacilli that are normal inhabitants of the intestinal tract. The most common is
Escherichia coli, followed by the
genera Proteus, Klebsiella and Enterobacter.
Among Gram positive bacteria are Enterococcus
fecalis and species of the genus Staphylococcus.
However, virtually all other bacterial and fungal agents can also cause urinary
tract infection (UTI) [16].
CONCLUSION
Inflation tests are internationally standardized and have great importance in the factor of evaluation of the product. According to World Health Organization specifications and condom quality standards, insufflation tests, integrity of the primary packaging and the amount of lubricant together are useful for evaluating condom performance. These properties are the best indicators of product performance. The insufflation tests measures both properties and is performed on most of the product with proven sensitivity in the relationship between these parameters and the degradation of the product. Hereafter, the results found, although the samples are within the limits of conformity established by the standards, illustrates the importance of carrying out the quality analyzes in a wide way, since, in most cases, the manufacturing lot indicates nonconformity in a or more tests, allowing to indicate the need, besides the certification, Sanitary Surveillance action on the product in the market. The bacteria found are among the dominant etiological agents responsible for more than 85% of cases of urinary tract infection, including Gram-negative bacilli that are normal inhabitants of the intestinal tract. The most common is Escherichia coli, followed by the genera Proteus, Klebsiella and Enterobacter. Among Gram positive bacteria are Enterococcus faecalis and species of the genus Staphylococcus. However, virtually all other bacterial and fungal agents can also cause urinary tract infection (UTI). In the microbiological evaluation, the results indicate the need to include, as acceptance criteria, the presence or not of pathogenic microorganisms, since the tests are complementary and are not necessarily correlated. This study reinforced the importance of monitoring the actions of this product in world trade [11,17-19].
1. Scaramuzza MC (2014) DST’S. Publicado por: http://www.boasaude.com.br/artigos
2. Nichiata LYI, Bertolozzi MR, Takahashi RF,
Fracolli LA (2008) The use of the “vulnerability” concept in the nursing area.
Rev Latino-am Enfermagem 16: 923-928.
3. ORGANIZAÇÃO PAN-AMERICANA DE SAÚDE (OPAS)
(2010) Módulos de Princípios de Epidemiologia para o Controle de Enfermidades.
Módulo 3: medida das condições de saúde e doença na população/Organização
Pan-Americana da Saúde. Brasília. Organização Pan-Americana da Saúde:
Ministério da Saúde. 94p: il. 7 volumes. ISBN 978-85-7967-021-3.
4. Bertolozzi MR, Nichiata LYI, Takahashi RF,
Ciosak SI, Hino P, et al. (2009) The concepts of vulnerability and adherence in
Collective Health. Rev Esc Enferm USP 43: 1320-1324.
5. Bryn N (2018) Four emerging STDs that you
can't afford to ignore. Mosaic.
6. Strelow VL, Vidal JE (2013) Invasive
meningococcal disease. Arq Neuro-Psiquiatr 71: 653-658.
7. Costa EA (2004) Vigilância sanitária:
proteção e defesa da vida. São Paulo: Sobravime.
8. Costa EA (2009) Vigilância Sanitária: temas
para debate. Salvador: EDUFBA.
9. AGÊNCIA NACIONAL DE VIGILÂNCIA SANITÁRIA
(2014) Resolução RDC nº 62, de 03 de setembro de 2008. Estabelece os requisitos
mínimos a que devem obedecer aos Preservativos Masculinos de Látex de Borracha
Natural. Brasília, 2008. Available in: http://azt.aids.gov.br/documentos/inventario/RDC%20n%C2%BA%2062-ANVISA.pdf
10. (2002) International Organization for
Standardization. ISO 4074: Rubber Condoms. Switzerland.
11. (2015) International Organization for
Standardization. ISO 4074:3: Natural latex rubber condoms - Requirements and
test methods. Switzerland.
12. Tremelling J, All A, Lleras L, Cancel A,
Jenkins D, et al. (2019) Poor quality male latex condoms found in Dominican
Republic: Quality assurance evaluation and public health impact. PLoS One 14:
e0210150.
13. Enersol Consulting Engineers (2010)
EInflation 3. Sidney, 2006. Biological METHODS. In: FARMACOPÉIA BRASILEIRA, 5
Edn. São Paulo: Atheneu.
14. (1999) International Organization for
Standardization. ISO 2859-1: Sampling procedures for inspection by attributes –
Part 1: Sampling schemes indexed by acceptance quality (AQL) for lot-by-lot
inspection. Switzerland, p: 87.
15. (2010) Biological methods. In: FARMACOPÉIA
BRASILEIRA, 5th Edn. São Paulo: Atheneu.
16. Robbins SL, Rim IN (2000) Patologia Estrutural
e Funcional. 6 Edn. Rio de Janeiro: Ed. Guanabara. Koogan, pp: 834-892.
17. (1997) American Society for Testing and
Materials. ASTM D 1976: Standard specification for rubber – Concentrated,
ammonia preserved, creamed and centrifuged natural latex. Philadelphia, p: 12.
18. Gerofi J, Shelley G, Donavan B (1991) A Study
of the relationship between tensile testing of condoms and breakage in use.
Contraception 43: 177-185.
19. Word Health Organization (2013) Effectiveness
of male latex condoms in protecting against pregnancy and sexually transmitted
infections. Geneva, Available at: http://www.who.int/mediacentre/factsheets/
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Alcoholism Clinical Research
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)
- Ophthalmology Clinics and Research (ISSN:2638-115X)