16415
Views & Citations15415
Likes & Shares
An experimental study was conducted from November 2015 to May 2016 at Aklilu Lemma Institute of Pathobiology, Addis Ababa University, Addis Ababa and College of Veterinary Medicine and Agriculture, Addis Ababa University, Bishoftu on the in vitro antibacterial sensitivity test of selected medicinal plants. The study was carried out with the objective of determining and comparing the in vitro antibacterial effect of 80% ethanol and methanol extract of Calpurnia aurea leaf (“Digita”) and Cordia africana root (“Wanza”) at various concentrations against Staphylococcus aureus, E. coli and Salmonella species by using agar disc diffusion method on Muller-Hinton agar and broth serial micro dilution methods with the help of resazurin. The plant materials were collected from Bishoftu. The mean zone of inhibition results showed that all of the extracts were active against tested bacteria. A wider mean zone of inhibition was demonstrated by ethanol extract of Calpurnia aurea leaf against Staphylococcus aureus that showed 15.20 ± 1.3; mean ± S.D followed by methanol extract was showed, 14.20 ± 2.04 at a concentration of 200 mg/ml. Both ethanol and methanol extracts of this plant at concentration of 200 mg/ml have showed 13.60 ± 1.34 and 13.60 ± 1.14, respectively against E. coli. The results also indicated that the root of C. africana inhibits the tested organisms. Moreover, ethanol extract of 200 mg/ml against E. coli showed wider inhibition zone of 12.60 ± 2.14 than S. aureus 11.20 ± 2.168 and Salmonella species 11.0 ± 1.58. Furthermore, each plant extract was tested with broth serial micro dilutions and the obtained concentration was ranged from 25 mg/ml to 0.78 mg/ml against tested bacteria. Besides this, the mean of higher MIC of C. aurea showed 2.35 mg/ml ± 0.91 against Staphylococcus aureus and Cordia africana against Salmonella species had (9.38 mg/ml ± 3.61). In conclusion, most of the extracts have shown considerable activities against test bacteria. These may justify the traditional uses of these two plants have the potential for discovery of novel antimicrobial agents from medicinal plants. Therefore, further study is required to isolate and identify as well as to assess the bioactive ingredients responsible for this effect at in vitro and in vivo level.
Keywords: C. aurea, C. africana, E. coli, In vitro anti-bacterial effects, Medicinal plant, Salmonella species, S. aureus
INTRODUCTION
The most important group of bacteria causing calf diarrhea are Escherichia coli (E. coli), Salmonella species and Clostridium perfringens. E. coli occurs as normal flora in the gastrointestinal tract of humans and animals. Pathogenic variants cause intestinal and extra-intestinal infections, including gastroenteritis, urinary tract infection, meningitis, peritonitis and septicemia [12].
Salmonellosis in dairy calves has many impacts on animal and human health that are considered as a major worldwide problem. Substantial economic losses were manifested through mortality and poor growth of infected animals as well as it has potential for zoonotic transmission [31].
Bovine mastitis has remained the most economically damaging disease that severely reduces milk production and often difficult to treat due to antimicrobial resistance [16]. In addition to heavy losses in milk quality and quantity, it is also causes irreversible damage to the udder tissue and less occasional fatalities [27]. The most important major pathogens that cause bovine mastitis are Staphylococcus aureus, Streptococcus uberis, Streptococcus agalactiae, Streptococcus dysgalactiae, Escherichia coli and Klebsiella species [25]. From these organisms, Staphylococcus aureus (S. aureus) is a major problem associated with milk causing diseases of humans and animals [19].
Antimicrobial agents are essential for the maintenance of health and welfare in animals as well as humans. However, the use of antimicrobials can be linked to the emergence and increasing prevalence of antimicrobial resistant bacteria. In addition to this, the development of antimicrobial resistance in bacteria of animal origin reduces the efficacy of veterinary antimicrobial drugs [34]. These bacteria are characterized by great resistance to antibacterial drugs, either by their natural resistance or by the development of different defense mechanisms against the host response [28]. Hence, the development of antimicrobial-resistance imposes the need to search for new effective drugs to circumvent the problem. Thus, there has been a resurgence of interest recently in the development of drugs from plants, especially from those of the developing countries that have a rich heritage of botanical ethno-pharmacopoea [20].
Traditional medicinal plants are widely used in different part of the world for curing diseases. They have maintained their popularity in developing world. These medicinal plants are also rapidly spreading in the industrialized countries [35]. In the developing countries, many people rely on traditional healing practices and medicinal plants for their daily healthcare needs. In some Asian and African countries, 80% of the population depends on traditional medicine for primary healthcare. This is because traditional medicine is a more affordable and accessible health care system when compared to modern medicine [5]. The acceptance of traditional medicine as an alternative form of health care and the development of microbial resistance to the available conventional antibiotics have led researchers to investigate the antimicrobial activity of herbal extracts [9].
In Ethiopia, traditional remedies represent not only part of the struggle of the people to fulfill their essential drug needs but also they are integral components of cultural beliefs and attitudes [1]. The Ethiopian flora is estimated to contain between 6500 and 7000 species of higher plants of which about 12% are endemic. More than 95% of traditional preparations in the country are of plant origin [29]. Despite their vital role in catering for the health of human and livestock population, large part of the knowledge of ethno-medicinal plants is on the verge of irreversible loss and declining to deterioration due to oral passage of herbal heritage from generation to generation verbally rather than in writings [32]. Consequently, the majority of those raising stock in rural areas are far from site of veterinary stations so that applying veterinary herbal medicine is the only option they have [7]. Concerns have risen about drug-resistant pathogens originating from an animal related source (food borne infections, direct contact) as well as bacteria of human origin acquiring resistance genes through gene transfer from these organisms and causing infections that are difficult to treat. The rapid emergence of multiple drug resistance strains of pathogens to current antimicrobial agents has generated an urgent intensive for new antibiotics from medicinal plants. Many medicinal plants have screened extensively for their antimicrobial potential worldwide [22].
Medicinal herbs have been used in one form or another under indigenous systems of medicine [13]. A range of medicinal plants with anti-microbial properties has been widely used by traditional healers. Among these plants, leaf of Calpurnia aurea (local name “Digita”) has been used In Ethiopia. Traditionally, the leaf of Calpurnia aurea are used for the treatment of syphilis, malaria, rabies, diabetes, hypertension, diarrhea, leishmaniasis, trachoma, elephantiasis, fungal diseases and different swellings, stomach-ache, bowel and bladder disorders [30,33]. Different parts of Cordia africana (local name “Wanza”) are used for skin disease, wound, diarrhea and ascaris infection in human and animals [37]. Although, there are few studies that evaluated the antimicrobial activity of C. aurea and C. africana, more experiments are needed to discover new antimicrobial agents and thus, recent information is important to evaluate the antimicrobial activity of medicinal plants.
Therefore, the objectives of this study were:
· To determine the in vitro antimicrobial effects of leaf extracts of Calpurnia aurea (Digita) and root extracts of Cordia africana (Wanza);
· To determine the minimum inhibitory concentration of each plant extracts, and;
· To evaluate the efficacy of selected medicinal plants on bacterial isolates.
MATERIALS AND METHOD
Study areas
A study of an in vitro antimicrobial effect of Phytopreparations was carried out from November 2015 to May 2016 in Bishoftu. The two plant materials were collected from Bishoftu (Debrezeit) and were identified at Aklilu Lemma Institute of Pathobiology (ALIPB), Addis Ababa University, (AAU). Bishoftu is located at 47 km South-east of Addis Ababa. The area has an altitude of 1,860 m above sea level with an average annual rain fall of 866 mm. It has a bimodal rainy seasons; a main rain season extends from the month of June to September and a short rainy season from March to May. The annual average minimum and maximum temperature is 11°C and 26°C, respectively. Day length is constant throughout the year (12-13 h) with about 6 h of sunshine during the rainy season and 8-10 h for the rest of the year. Humidity is about 50.9% [24].
Study design
The study design was an experimental design of anti-microbial efficacy with extract of medicinal plants. During the study period two different medicinal plants namely, Calpurnia aurea leaf (local name “Digita”) and Cordia africana root (Local name “Wanza”) were selected for their in vitro inhibitory effect against Salmonella species, E. coli and S. aureus and for those with the highest antibacterial activity on disc diffusion assay in which MIC was determined. Finally, the antibacterial activities of two solvent extracts with high efficacy were compared with standard antibiotics namely; streptomycin for E. coli and Salmonella species and tetracycline for S. aureus.
Description of study plants and their uses
Calpurnia aurea (“Digita” in Amharic): It belongs to the group of flowering plants within the family Fabaceae. It is a shrub or small tree growing in or along the margin of forests in many parts of Ethiopia [4]. In Ethiopia, the leaf of C. aurea is traditionally used for the treatment of syphilis, malaria, rabies, diabetes, hypertension, diarrhoea, leishmaniasis, trachoma, elephantiasis, fungal diseases and different swellings, stomachache, bowel and bladder disorders [33].
Cordial africana (“Wanza” in Amharic): It is belongs to the family of Boraginaceae; which is a small to medium-sized evergreen tree, 4-15 m high, heavily branched with a spreading umbrella-shaped or rounded crown. The bark is grayish-brown to dark brown, smooth in young trees, but soon becoming rough and longitudinally fissured with age; young branchlets with sparse long hairs. Leaves are broad thinly dark green. It grows at altitude of 550-2,600 masl and mean annual rainfall 700-2,000 mm large leafed Cordia thrives in forest soil. Mature fruits have a sweet, mucilaginous, edible pulp [18].
Bacterial species used for the experiment
Bacterial species used in the study consisted of three pathogenic bacteria namely Salmonella species and E. coli isolates obtained from calves with diarrhea and S. aureus was from bovine mastitis. These organisms cause food borne diseases in human and diarrhea and mastitis in animals. Their effect on public health is high. Moreover, they have proven to be ideal for screening studies since it is easy to culture and generally present in large numbers in animal feces and milk. These bacteria streaked onto nutrient agar to obtain pure isolated colonies following a standard aseptic technique and the four-way streak plate inoculation [8].
Study procedures
Collection of plant materials and pre extraction preparation: The fresh leaves of Calpurnia aurea and roots of Cordia africana were collected from Bishoftu. The preparation of the plants and extractions were carried out following a protocol described by Gemechu et al. [15]. After collection, the plants were washed with tap water to eliminate any foreign matter. Each plant material chopped into small pieces, air-dried under shade and grounded to fine powder using a wooden pestle and mortar. The material sieved and weighed before maceration.
Preparation of crude extracts: The grounded leaves and roots were weighed to be 100 g using an electronic balance and the powder was put into beaker. 400 ml (1:4 ratios) of 80% ethanol was added and macerated for 72 h with automatic shaker and the solution was filtered using a conical flask, a filter funnel and Whatman No. 1 filter paper (Camlab, UK). Filtered extract poured in a 500 ml round bottom flask. The solvents were evaporated from the filtered extracts in Rota vapor. Temperature of the water bath in the Rota vapor was set at 60°C to remove the remaining solvent. This temperature used because the evaporation under reduced pressure makes it possible to evaporate at much lower temperatures. Same procedure was repeated with 80% methanol for each plant.
The dried methanolic and ethanolic extracts obtained from each plant were taken out, put in petridishes and kept in a dry oven at 40°C to remove the remaining solvent until all solvents were evaporated. To determine the yielded quantities of extracts in two solvents, the weight of petridishes were subtracted from the weight of the petridishes and samples. The percentage weight yields were calculated as described by Emtinan et al. [14].
% yield = (weight of extract obtained)/ weight of plant powder
Preparation of antimicrobial discs from extracts: Preparation of paper disks: Paper disks with approximate diameter of 6 mm were punched out one by one from a sheet of paper Whatman Filter paper #1 using an ordinary office two-hole puncher. Precautions were taken to avoid overlapping of holes. Before the discs were impregnated they were kept on aluminium foil and autoclaved a 121°C for 15 min [10]. A stock solution of 200 mg/ml (20%) in 2% Tween 80 was made for each extract. Concentration was recorded on a weight by volume (w/v) basis. Stock solution was stored in regular refrigerator set at +4°C. Working solutions made from the stock solution. To prepared 20% (20 g/100 ml), taken 1 g of crude extract and it was mixed with 5 ml of 2% Tween 80 by using vortex. Then, 2.5 ml from the first test tube (20%) was transferred to a second test tube by using micropipette to prepare 100 mg/ml (10%). The procedure continued by transferring 2.5 ml of solution taken from the 10% to prepare 50 mg/ml (5%) and added 2.5 ml 2% Tween 80 to the third test tube then mixed by using vortex. Reconstituted extracts were prepared for the evaluation of antimicrobial properties against the bacteria.
This was done by taking 20 µl of each extract of various concentrations and impregnated at 6 mm blank paper disc and leaving them in the biological hood (LABCONCO Purifier Class II bio-safety cabinet; Kansas, Missouri) to dry [11]. After dried they were used for screening the antibacterial activity. Tween80 (2%) was used as negative control while standard antibiotic discs were used for Salmonella species and E. coli (streptomycin) and for S. aureus (tetracycline) as a positive control. The medicinal plants were chosen based on their frequent use for the treatment of those organisms.
Preparation of the test bacteria: The plant extracts were tested against E. coli and Salmonella species obtained from diarrhea of calves while S. aureus from bovine mastitis. These bacterial isolates obtained from the Department of Microbiology, College of Veterinary Medicine and Agriculture. The bacterial isolates were maintained on nutrient agar and sub cultured every three days. Each bacterial isolates was suspended in 5 ml of saline solution and adjusted to give a concentration of bacterial cells equivalent to 0.5% McFarland turbidity standard prior to the antibacterial testing [11].
Determination of in vitro antibacterial susceptibility
Principle: When a filter paper disc impregnated with a chemical placed on agar medium, the chemical will diffuse from the disc into the agar. Multiple factors can alter the size of the no-growth-zone around the disc. The solubility of the drug in question, its molecular size and its antimicrobial potency will be determined. If an organism is placed on the agar medium suitable for its growth, it will not grow in the area around the disc, if it is susceptible to that antibacterial agent. If there is no growth around the disc, it is known as ‘zone of inhibition’.
Procedure: Muller-Hinton agar (38 g) (Biotech, UK) medium was used for antimicrobial sensitivity test, and was mixed with 1litter of distilled water boiled to dissolve completely and autoclaved at 121°C for 15 min. Mueller-Hinton agar medium was prepared by pouring sterile agar plates and left to set. The agar plates incubated for 24 h at 37°C to confirm their sterility. When no growth occurred after 24 h, the plates considered sterile and used for antimicrobial sensitivity test [3]. The colonies of the same morphology were scooped using a wire loop from the nutrient agar and mixed using sterile normal saline, and agitated with a vortex mixer. The turbidity of the bacterial suspension adjusted by comparing with 0.5 McFarland turbidity standards (1.5 × 108 CFU/ml) [24]. The standard and the test suspension were placed in a 10 ml sized test tubes and compared against a white background with contrasting black lines until the turbidity of the test suspension equates to that of the turbidity of the standard. Adjustments of the turbidity made by adding saline or colonies depending on the degree of turbidity.
For each bacterium, a sterile applicator swab was dipped into its standardized cell suspension and squeezed gently by rotating the swab against the inside of the glass tube above the liquid to remove the excess fluid. The entire surface of each agar plate inoculated by streaking the swab in different directions to ensure a uniform growth. Additional plates for each organism were incubated by this fashion. A sterile disposable syringe needle used to pierce in the centre of a plain filter paper disc. The syringe was held upright with disc above. The required concentration of the plant extraction taken by a micropipette and discharged gently on the disc until the disc was thoroughly soaked. Then, the disc stamped on the surface of Mueller-Hinton agar medium. Changing the tips of micropipette, the processes were repeated [10]. All the discs placed approximately the same distance from the edge of the plate and from each other.
The inoculated plates kept inverted in incubator at 37°C for 24 h. After 24 h, the plates viewed against a black background and illuminated with reflected light and zones of inhibition measured by ruler and recorded. The experiment repeated for five times and the result expressed as average value of zone of inhibition of each plant [11].
Determination of minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) is the lowest concentration of an antimicrobial that will inhibit the visible growth of a microorganism after overnight incubation. The micro dilution broth method was used to determine the MIC [21]. Microorganisms were tested for their ability to produce visible growth on a series of agar plates at different concentrations of crude extract with incubation at 37°C for 18 h. The lowest concentration of crude extract that inhibited the visible growth of microorganisms designed as the MIC of that agent. The 80% ethanol and methanol leaf extract of C. aurea and root extract of C. africana inhibited growth of microorganisms were tested for MIC. The working solution of extracts (50 mg/ml) were diluted out across a 96-well in a serial dilution to give final testing concentrations of 50, 25, 12.5, 6.25, 3.12, 1.56, 0.78, 0.36, 0.18 and 0.09 mg/ml. The first column (C1) were filled by 200 µl of working solution (50 mg/ml) then, 100 µl of tryptan soy broth was added into columns 2-10 (C1-C10). Columns 11 and 12 were served as negative control and positive control wells. 100 µl extract were taken from C1 and added to C2, mixed thoroughly and transferred into each well up to C10..
Each bacterium in trypsin soy broth was adjusted to 0.5% McFarland contain approximately 1.5 × 108 CFU/ml and then 100 μl bacteria added to each well. A bacterium with media was used as a negative control in the C11 and media with solution of Streptomycin for Salmonella species and E. coli, while tetracycline for S. aureus as positive control in the C12. 96-well micro plate incubated at 37°C for 18 h. After 18 h, 2% solution of 20 μl resazurin added to each well and re-incubated for 2 h and color change was recorded. The MIC defined as the lowest concentration of the extracts that prevented a color of resazurin to be changed from blue to pink (visual determination) [26].
DATA ANALYSIS
All the data obtained from the experimental result entered into Excel Microsoft office and SPSS version 20 statistical software was used for data analysis. The experimental results expressed as mean ± standard deviation of five replicates of zone of inhibition and four replicates of MIC presented.
RESULTS
Percentage yield of extract Calpurnia aurea and Cordia africana
A greenish, shiny and jelly-like stock was obtained from 80% methanolic and ethanolic leaf extracts of C. aurea and a yellowish brown and shiny stock was obtained from C. africana by using the same solvents as C. aurea. The following results indicated that, methanol solvent in this study was the best solvent with a yield of 7.62% of extract from 100 g powder of C. aurea followed by ethanol, which was 5.72% with the same weight of powder. 4.76% yield was obtained from 100 g methanol extract of C. africana and 3.73% yield was obtained from 100 gram ethanol extract from the same plant.
The effects of crude extracts on selected bacterial zone of inhibition
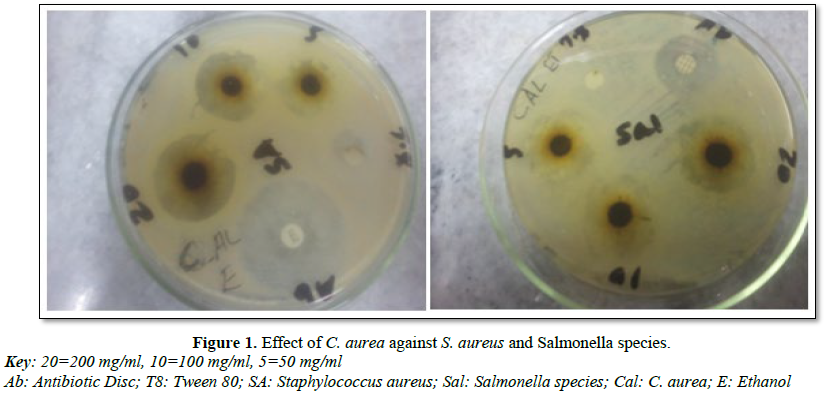
Each plant extracts were tested at different concentration levels (20%, 10% and 5%) and these two plant extracts showed strong anti-microbial activity mainly at higher concentration against S. aureus, E. coli and Salmonella species which is expressed by mean diameter zone of inhibition and standard deviation (Tables 2 and 3). The 80% ethanol and methanol extract of the leaf C. aurea were highly inhibited the growth of the three tested bacterial species (Table 2 and Figure 1). C. aurea showed in vitro antimicrobial activity at a concentration of 200 mg/ml against S. aureus, with ethanol (15.20 mm ± 1.3; Mean ± S.D) and methanol (14.20 ± 2.04) extracts. On this bacteria, the standard tetracycline on ethanol and methanol extract showed (12.6 ± 1.67 and 13.6 ± 1.52), respectively. Both ethanol and methanol extracts of this plant at concentration of 20% have showed 13.60 ± 1.34 and 13.60 ± 1.14, respectively against E. coli. Standard streptomycin showed (11.40 ± 1.51 and 10.40 ± 2.07) on ethanol and methanol extraction against E. coli.
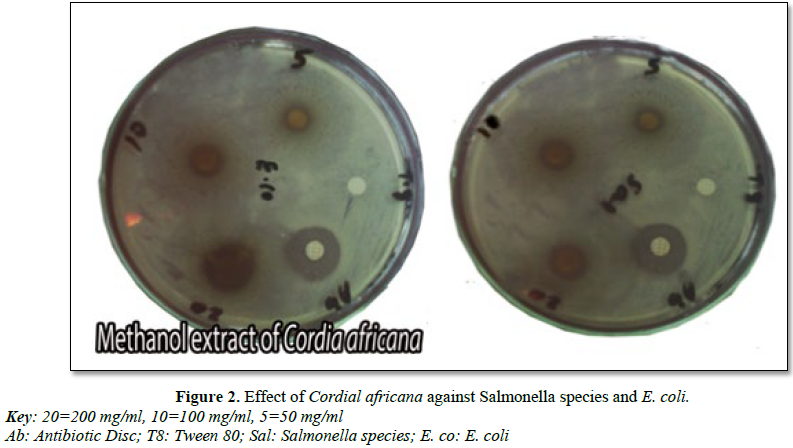
It was also indicated that, the root of C. africana has showed inhibition against the tested organisms (Table 3 and Figure 2). MZI ethanol extract of 200 mg/ml against E. coli species has showed wider inhibition (12.60 ± 2.14) followed by S. aureus (11.20 ± 2.168) and Salmonella species (11.0 ± 1.58). Moreover, methanol extract of this plant showed zone of inhibition against all of test organisms.
Minimum inhibitory concentration (MIC)
DISCUSSION
The present study was conducted to investigate the antimicrobial activity of two plant extracts namely: C. aurea and C. africana. The 80% ethanol and methanol extracts of these plants were screened for their antimicrobial activity against E. coli, S. aureus and Salmonella species.
The findings of the present study demonstrated that the ethanol extract of C. aurea has showed remarkable antibacterial activity followed by methanol extract. 200 mg/ml ethanol and methanol extracts, impregnated discs in S. aureus (15.20 ± 1.30 and 14.02 ± 2.04) were wider than the diameter of conventional antibiotic disc of tetracycline (12.60 ± 1.67 and 13.40 ± 1.52) while the 100 mg/ml of this extract was comparable to that of standard tetracycline. Similarly, Adeolu et al. [2] also reported that C. aurea leaf against S. aureus, E. coli and Salmonella species showed good antimicrobial activity which agreed with this study.
The MZI ethanol and methanol extracts of C. aurea against E. coli at the concentration of 200 mg/ml was 13.6 ± 1.34 and 13.60 ± 1.14, respectively. This result was also higher than the antibiotic disc streptomycin (11.40 ± 1.51 and 10.40 ± 2.07). In addition to this, the crude extracts of these two solvents have good efficacy on Salmonella. Despite this fact, the 20% concentration has been less than the standard streptomycin. The 2% Tween 80 impregnated discs used, as negative control have not showed any inhibition against the test organisms, which implies that the inhibition observed by the bioactive ingredient of the plant. Binyam et al. [6] reported in his experiments that C. aurea in different bacteria including E. coli has inhibitory effect and the MZI of E. coli in his experiment were 13.3 mm, which supports the current study.
The value of methanol extract from C. aurea leaf against S. aureus showed 14.20 ± 2.04. This result was also higher when compared to the 11 mm finding obtained by Shemsu et al. [30]. Moreover, in his experiment, the value of E. coli (14 mm) was comparable with this study (13.6 ± 1.34).
The current finding also revealed that C. africana root at different concentrations has moderate mean zone of inhibition when compared with C. aurea. The MZI with ethanol extract of C. africana against E. coli at the concentration of 200 mg/ml has showed higher value (12.60 ± 1.14) than the standard streptomycin (9.6 ± 1.14). In addition, this plant had good inhibitory effects on E. coli than Salmonella (11.20 ± 2.168) and S. aureus (11.0 ± 1.58). 20% and 10% ethanol extracts showed better anti-microbial activity than methanol extract of the same plant against S. aureus and E. coli. In this study, the effect observed by the ethanol extract of C. aurea leaf and C. africana root on tested bacteria showed a good inhibitory effect in all concentrations than methanol extract except Salmonella species. From this result, it is possible to hypothesize that different ingredients of the plant/herb have different solubility depending on the solvents used. Hence, ethanol solvent has been found with better zone of inhibition than methanol.
According to the previous study, different parts of C. africana for instance, its bark, leaf, stem and fruit had been studied for antimicrobial effect on different pathogenic bacteria and fungi and exhibited effects against most of the tested organisms [14]. In this study methanol extract of C. africana root against S. aureus and E. coli was found with narrow mean zone of inhibition when compared the study of Emitinan et al. [14] which worked on the stem and bark of this plant while the result obtained by leaf extract of their study was comparable to the result obtained from root extract of this study. Moreover, the results of the current study showed that methanol extract of C. africana root were with good mean zone of inhibition against S. aureus and E. coli that disagreed with the previous works done by Gebregergs et al. [14] who reported that methanol extract with the leaf of this plant has no zone of inhibition. This variation and similarity may be due to the different bioactive ingredients of this plant part, method of sensitivity test performed and quality of the bacterial isolates.
Inhibition of bacteria at all concentrations against S. aureus, Salmonella species and E. coli isolates were recorded. Although the comparison among these test materials suggested that the herbal preparations do have a capacity to inhibit the growth of test organisms with a similar or a different manner to that of conventional antimicrobial agents, there are no established standard formulae to judge the level of zone of inhibition as resistant, intermediate and susceptible for phytopreparations [16].
Minimum inhibitory concentration of the leaf of C. aurea and the root of C. africana were tested repeatedly for four times against Salmonella species, E. coli and S. aureus and they showed different level of activities. Ethanol extract of C. aurea MIC against S. aureus has showed 2.35 mg/ml ± 0.91 and this result is higher than the result obtained from E. coli (4.69 ± 1.8) and Salmonella species (7.42 ± 5.9) that inhibited at lower concentration. Both methanol and ethanol extracts of this plant have showed inhibitory activity against S. aureus and E. coli at the same concentration (4.69 mg/ml ± 1.8), respectively. Adeolu et al. [2] reported that the MIC of these three bacterium inhibited by 5 mg/ml concentration that disagreed with the current study by concentration of 2.35 mg/ml ± 0.91 and 4.69 mg/ml ± 1.8 against S. aureus and E. coli, respectively. In case of Salmonella species, the minimum inhibitory concentration on this study was lower than Adeolu et al. [2] (7.42 mg/ml ± 5.9). According to this finding, ethanol extract of C. aurea showed better inhibitory activities at a lower concentration than that of methanol extract. Besides this, S. aureus was more susceptible for this extract followed by E. coli and Salmonella species.
Ethanol extract of C. africana inhibited Salmonella species at lower concentration (9.38 mg/ml ± 3.61) followed by S. aureus (14.1 mg/ml ± 7.86) and (15.63 mg/ml ± 6.25). Among tested organisms, ethanol extract against S. aureus and methanol extract against Salmonella have showed inhibitory activity at the same concentration (14.1 mg/ml ± 2.04). The higher inhibitory activity that was obtained from methanol extract against Salmonella species with 14.2 ± 2.04 and S. aureus showed the least mean of MIC (21.9 mg/ml ± 6.25).
In general, these medicinal plants have showed promising anti-microbial activity against these tested organisms. These medicinal plant chemical classes such as tannins, terpenoids, alkaloids, polyphenols that been reported to have antimicrobial activity. The variation of efficacy among these phytopreparations against the bacteria could be attributed to the way of plant preparation, season of collection, stage of the plant, place of collection, way of drying, means of extraction, the solvent used, preservation or storage of the extract till evaporation, amount of bacterial swab streaked on the agar plates and other unnoticed factors.
CONCLUSION AND RECOMMENDATIONS
As antibiotic use increases in veterinary medicine, the issue of bacterial resistance to antimicrobial therapy becomes more worrisome. Losses due to diarrhoea and clinical mastitis cases are frustrating the dairy industry. Furthermore, the predisposing factor of diarrhoea and mastitis has a potential, which become a subject of public health concern. The commonly used antibiotic is increasing from day to day. However, the use of antimicrobials can be linked to the emergence and increasing prevalence of antimicrobial-resistant bacteria. According to this, the new drug needed to sensitize from those plant which had antimicrobial effect. African plants have long been the source of important products with therapeutically value. Despite the great role of traditional medicine and medicinal plants in the primary health care, most of the claims by traditional healers are that it is not scientifically investigated in the developing country including Ethiopia. C. aurea and C. africana extracted with ethanol and methanol demonstrated good antibacterial activities at concentrations tested which would justify their traditional use. Both ethanol and methanol extracts of C. aurea and C. africana showed a good inhibitory antibacterial activity. From the finding of this study, it is possible to conclude that C. aurea and C. africana showed good antibacterial activities. The obtained results were effects on three bacteria in different concentrations and the result showed a potential antibacterial activity against Salmonella species, E. coli and S. aureus. The increase in prevalence of multiple drug resistance has shown the development of new synthetic antimicrobial and different diseases. Moreover, it is necessary to search for the new drugs from plant sources with new antimicrobial effects and acts upon different infectious diseases.
Therefore, from the findings of this study the following recommendations are forwarded:
· Studies should be conducted to identify and isolate the active compounds responsible for the observed antimicrobial property for C. aurea and C. africana.
· In vivo effectiveness of medicinal plants should also be evaluated.
· Conservation priority should also be given to medicinal plants for more diversified medicinal uses.
· Toxicity studies of the active plant principle should be done to determine their safety.
ACKNOWLEDGMENT
1. Abebe D, Debella A, Urga K (2003) Medicinal plants and other useful plants of Ethiopia. Camerapix Publishers Int Singapore, pp: 54-61.
2. Adeolu A, Florence O, Srinivas K, Anthony J, Patrick J, et al. (2008) Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement Altern Med 8: 53.
3. Aibinu E, Odunayo R, Akinsulire I, Ibukun E, Tayo A, et al. (2007) In vitro antimicrobial activity of crude extracts from plants Bryophyllum pinnatum and Kalanchoe crenata. Afr J Tradit Complement Altern Med 4: 338-344.
4. Asres K, David P, Polo M (1986) Two novel minor alkaloids from Ethiopian C. aurea. Planta Med 25: 302-304.
5. Bhushan P, Dnyaneshwar W, Pushpangadun P, Narendra B (2005) Ayurveda and traditional Chinese medicine, a comparative overview. Evid based Complement Altern Med 2: 465-473.
6. Binyam A, Getachew T, Nigatu K, Wondu M, Simenew K (2014) Potential in vitro anti-bacterial action of selected medicinal plants against Escherichia coli and three Salmonella species. Int J Microbiol Res 5: 85-89.
7. Brhanemeskel W, Tefera A, Muthuswamy R (2008) Ethnoveterinary use of veterinary plants by traditional healers in Dabat District, north western Ethiopia. Phcogmag 4: 24-37.
8. Cappuccino J, Sherman N (2008) Microbiology, a laboratory manual. 8th Edn. Pearson/Ben-jamin Cummings, San Francisco.
9. Chukwuka K, Ikheloa J, Okonko I, Moody J, Mankinde T (2011) The antimicrobial activities of some medicinal plants on Escherichia coli as an agent of diarrhea in livestock. Adv Appl Sci Res 2: 37-48.
10. Clinical and Laboratory Standards Institute (CLIS) (2010) Performance standards for antimicrobial susceptibility testing. 20th Informational Supplement.
11. Clinical Laboratory and Standard Institute (CLSI) (2014) Performance standard for antimicrobial susceptibility testing. 24th Information Supplement.
12. Daniel A, Shaohua Z, Emily T, Sherry A, Aparna S, et al. (2012) Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States. J Center Control Prev Dis 18: 5.
13. Dubey N (2004) Global promotion of herbal medicine, India’s opportunity. Curr Sci 86: 37-41.
14. Emtinan A, Hassan H, Muddathir S, Afra A, Salwa G, et al. (2015) Antimicrobial and phytochemical screening of Cordia africana in Sudan. World J Pharm Res 4: 257-269.
15. Gemechu A, Giday M, Worku A, Ameni G (2013) In vitro anti-mycobacterial activity of selected medicinal plants against Mycobacterium tuberculosis and Mycobacterium bovis strains. BMC Complement Altern Med 13: 291.
16. Getahun K, Kelay B, Bekana M, Lobago F (2008) Bovine mastitis and antibiotic resistance pattern in ellale smallholder dairy farms, central Ethiopia. Trop Anim Health Prod 40: 261-268.
17. Getu A, Zemede A, Ensermu K (2016) Cordia africana (Boraginaceae) in Ethiopia: A review on its taxonomy, distribution and ethnobotany and conservation status. Int J Botany Stud 1: 37-45.
18. Geyid A, Abebe A, Debella Z, Mekinene F, Abera F, et al. (2005) Screening of some medicinal plants of Ethiopia for their antimicrobial properties and chemical profiles. J EthnoPharmacol 97: 421-427.
19. Girum F (2016) Antimicrobial Susceptibility of Staphylococcus aureus in cow milk, Afar Ethiopia. Int J Mod Chem Appl Sci 3: 280-283.
20. Hart C, Karriuri S (1998) Anti-microbial resistant in developing country. Br Med J 317: 647-650.
21. Irith W, Kai H, Robert E (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 6: 13.
22. Kaur G, Arora D (2009) Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med 9: 9-30.
23. Laloo D, Hemalatha S (2011) Ethnomedicinal plants used for diarrhea by tribals of Meghalaya, north east India. Pharmacogn Rev 5: 147-154.
24. Murray P, Baron E, Pfaller M, Tenover F, Yolken R, et al. (1999) Manual of clinical microbiology. 7th Edn. Washington, DC.
25. Olde R, Barkema H, Kelton D, Scholl D (2008) Incidence rate of clinical mastitis on Canadian dairy farms. J Dairy Sci 91: 1366-1377.
26. Primm T, Franzblau S (2007) Recent advances in methodologies for the discovery of anti-mycobacterial drugs. Curr Bioactive Compounds 3: 1-8.
27. Radostits O, Gay C, Blood D, Hinchcliff K (2000) A Text Book of the Disease of Cattle, Sheep, Pigs, Goats and Horses. 9th Edn. New York: W.B. Sounders Company Ltd., pp: 809-827.
28. Raul C, Mariangel S, Ribeiro M, Manoel E (2007) Determination of the minimum inhibitory concentration of four medicaments used as intracanal medication. Aust Endod J 33: 107-111.
29. Sebsebe D, Ermias D (2001) Basic and applied research in medicinal plants of Ethiopia. In Proceedings of the National Workshop on Conservation and Sustainable Use of Medicinal Plants in Ethiopia, Addis Ababa, pp: 29-33.
30. Shemsu U, Alemu T, Nigatu K (2013) Antidiarrheal and antimicrobial activity of C. aurea leaf extract. BMC Complement Altern Med 13: 21.
31. Smith K, Stenzel S, Bender J, Wagstrom E, Soderlund D, et al. (2004) Outbreaks of enteric infections caused by multiple pathogens associated with calves at a farm day camp. Pediatr Infect Dis J 23: 1098-1104.
32. Taddese T (2007) In vitro antimicrobial effects of combertum molle on Staphylococcus aureus isolates. Unpublished DVM thesis, AAU.
33. Tadeg H, Mohammed E, Asres K, GebreMariam T (2005) Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. J. Ethnopharmacol 100: 168-175.
34. Tamura Y (2003) The Japanese veterinary antimicrobial resistance monitoring system (JVARM). OIE International Standards on Antimicrobial Resistance, pp: 1-24.
35. World Health Organization (2003) Fact Sheet. Traditional Medicine, Geneva.
36. Yimer M, Gezheagne M, Biruk T, Dinaol B (2014) A review on major bacteria causes of calf diarrhea and its diagnostic method. J Vet Med Anim Health 7: 173-185.
37. Yonathan D, Yeshitila A, Tadesse M, Mathewos A (2015) Extraction and physico-chemical characterization of Cordia africana Lam seed oil. J Adv Botany Zoology 4: 8.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Astronomy and Space Research
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)