1224
Views & Citations224
Likes & Shares
Wasting is among the leading nutritional problems, causing morbidity and mortality in children less than five years of age when the nutrition of the child is principal markers of growth. Failure to fulfill micronutrient requirements, a challenging environment and the insufficient provision of care, are all factors liable for this situation that affects nearly 200 million children who are under 5 years of age. The timing and duration of nutritional disgrace lead to different physiological consequences. Wasting is however just one characteristic of a complex syndrome together with developmental delay, reduced cognitive function, impaired immune function and metabolic disturbances guiding to increase the prospective risk of non-communicable diseases in adulthood. Prevention is possible at all stages of the life cycle through the implementation of interventions and mainly includes the promotion of exclusive breastfeeding up to the age of 6 months and the introduction of complementary foods and family foods with enough micronutrient density. Treatment is possible, at least until the age of 5 years, and can guide the reversal of all symptoms, although more research is needed to explain whether the acceleration of the growth rate could also lead to an increased risk of metabolic syndrome.
Keywords: Child, Nutrition, Growth, Wasting, Diet, Micronutrient deficiencies
INTRODUCTION
Under nutrition is the most devastating problem affecting the majority of the world’s children [1]. That poor nutritional status during childhood has long-lasting scarring consequences [2]. Under nutrition diminishes the working capacity of an individual during adulthood [3] and it silently destroys the future socio-economic development of nations [2]. Ultimately, it causes the vicious cycle of intergenerational under nutrition [4]. Underweight, stunting, and wasting are the three main indicators used to define under nutrition [5], which is having a Z-score lower than two standard deviations as compared to the reference population of the same age and sex [6]. Low weight-for-height (WHZ ≤ −2 SD) is an indicator of wasting, which is generally associated with recent illness and child failure to gain weight [7]. Wasting occurs as a consequence of short-term response to inadequate intake or an infectious disease episode [8] and can be reversed if the child has access to adequate dietary intake in an environment that is free from infectious disease [9]. It is suggested, however, that although wasting is a short-term health issue, repeated episodes of it may lead to stunting [10,11]. This is perhaps what happens in many resource-poor settings, where dietary intake is consistently inadequate and infectious diseases are highly prevalent [9]. Worldwide, 52 million children under five years of age are wasted [12] and most of the global burden of wasting (acute under nutrition) is found in developing countries [7,13,14]. Likewise, the magnitude of the problem is substantial and persistent in Sub–Saharan Africa (SSA) [15]. Evidence-based health and nutrition findings have a crucial role in improving the level of wasting and mortality reduction in children [16].
Though information is available on the prevalence and factors associated with chronic and acute under nutrition in many countries, there is little information on the magnitude and factors that contribute to the development of wasting within children. That’s why in this paper I review the physiopathology of wasting and discuss the implications for programs and policy.
A HEALTHY CHILD
Achieving and maintaining optimal child health is a challenge for parents, health workers, pediatricians, nutritionists, public health specialists and those concerned with the health and well-being of the future generation. A healthy child is not only a child with adequate physical development, both in terms of achieved size and acquired motor skills, but also with an adequate neurological, psychological and emotional development. Optimal growth and development, therefore, encompass the whole of well-being – physical, psychological and social.
Furthermore, as we are increasingly aware of the relationship between current health and nutritional status and its impact on both immediate and future risk of disease, we should define a healthy child as one who is going to be a fit and healthy adult with low morbidity for chronic diseases, adequate physical work capacity and adequate reproductive performance.
Currently, it is estimated that malnutrition is responsible for 55% of all child deaths, but this is probably an underestimate [17]. The vast majority, about 70% of the children who are wasted live in Asia, especially in South-Central Asia [18] and, despite the fact that in some of the world regions there is a downward trend, in 2012 the estimated global prevalence of wasting was almost 8% [19].
PHYSIOPATHOLOGY OF WASTING
Changes in body composition during malnutrition
When the energy intake is insufficient to maintain metabolism, various physiological adjustments take place to ensure that key organs have a sufficient supply of fuel by drawing on the body's nutritional reserves, mainly fat and muscle [20]. If food deprivation is maintained and occurs during the growth period, animal models suggest that important changes in the relative size of the organ take place, avoiding the brain but affecting the heart, kidney, thymus and especially the muscles, with possible long term consequences even during adult life [21]. These adjustments follow rapid changes in insulin and glucagon levels and include both short-term and long-term regulation of key enzymes, which puts the body in an energy-saving mode.
During the acute absence of infection, the metabolism is maintained mainly by mobilization of fat reserves and most of the organs obtain the energy from fatty acid catabolism. Most fatty acids do not easily cross the blood-brain barrier, however, and the brain usually receives most of its energy from glucose. After a few days of insufficient energy supply, the brain shifts to also using water-soluble ketone bodies, which are also derived from the catabolism of fatty acids. This shift toward ketone bodies occurs earlier in children than in adults, presumably due to the higher relative brain mass of children, with a high glucose demand in relation to body weight. Even with the increasing use of ketone bodies, the brain (and red cells) continue to use glucose, which is produced from the glycerol derived from triglycerides, but also by the liver and kidney from amino acids (alanine and glutamine) released from muscle. Muscle amino acids are also necessary to maintain protein metabolism when protein intake is insufficient (which usually goes hand in hand with deficient energy intake). But in the absence of infection, only a minimal level of protein catabolism is required to fulfill glucose and protein needs, muscle mass loss is minimal, and the organism lives mainly on body fat stores. In this situation, death occurs when the fat supply runs out [20].
During infection, there is an added double nutritional stress. First, food intake is typically reduced as a result of anorexia, and second, there is an increased demand for amino acids for the accelerated synthesis of acute phase proteins, for the production of glutathione and for building up the adaptive immune response. These reactions to infection have a negative effect on nitrogen balance (even when dietary intake is adequate), which leads to the mobilization of amino acids from lean tissues, mainly from muscle [22].
The body’s response to a range of aggression can also shed light on the body’s response to restricted dietary intake and infection. Conditions as different as burns, sepsis and cancer are also associated with the mobilization of amino acids from muscle by mechanisms that are the same as those involved in responses to insufficient energy intake and infection [23]. Inflammation can lead to the development of insulin resistance, also contributing to a reduction of nutrients available for muscle metabolism [24]. When infection or inflammation is associated with a poor-quality diet and insufficient nutrient intake, these effects reinforce each other, leading to rapid deterioration of muscle mass.
In summary, the metabolic adjustments that occur during malnutrition lead to a decrease in fat and muscle masses, the latter being more important when there is associated inflammation or infection. These changes in body composition, which are indirectly reflected by the anthropometric indices of wasting, have important functional implications.
Decreased muscle mass in wasting—a plausible common link with increased mortality
Wasting have long been known to be associated with an increased risk of death [25,26], but the mechanisms of this association are rarely discussed. Anthropometric indices of wasting measure a statistical deviation of body size from a standard. A direct link between anthropometric indices and mortality would suppose that the organism is able to evaluate this deviation. However, the existence of a central “sizostat” allowing the organism to compare its current size with a theoretical value, postulated in 1963 by Tanner [27], has not been confirmed by experimental evidence and now seems very unlikely [28]. The existence of a physiological change related to this difference expressed in standard deviations of the growth standards seems even less plausible. Hence, z-scores for weight-for-height (indices of wasting) should be considered as statistical concepts with no clear link with physiological changes, and association with mortality should be considered as indirect and non-causal.
In his early papers, Waterlow speculated that children with wasting have an alteration of body composition. Forty years later, the most compelling explanation of the association of wasting with mortality is indeed that wasting reflect changes in body composition, in particular a decrease in muscle and fat mass, which, if severe, compromise the provision of fuel to vital organs, such as the heart, kidney, liver, immune system and gut, especially when infection is also present.
In clinical settings, muscle mass is a major determinant of survival in malnourished adult patients with infections [29] and also in conditions as diverse as liver transplantation [30], liver cirrhosis [31], cancer [32,33] and chronic obstructive pulmonary disease [34,35]. Studies in these patients consistently show that those with a high muscle mass have better survival, independently of their body mass index.
Children have a lower muscle mass in relation to body weight than do adults. This effect is difficult to quantify, as muscle mass cannot be measured reliably in infants and young children, but it is undoubtedly present, as post-mortem studies show that muscle mass represents only 23% of body mass in new-borns, versus 43% in adults [36]. Also, in children, the brain, with its high demand for glucose, has a size in relation to body weight that is unprecedented in the history of evolution. Since a large proportion of glucose is derived from amino acids derived from muscle, an effect of low muscle mass on survival is even more likely for children than for adults. This is also suggested by epidemiological studies linking indices of muscularity at the arm level with the risk of death in contexts where the association between infection and malnutrition is a major cause of death [37,38].
Despite the importance of muscle mass as a determinant of survival in the association between malnutrition and infection, and the visible muscle wasting seen in children with SAM, the decrease of muscle mass in child malnutrition has hardly been measured. Those studies which have been done took place in the 1970s. Limitations in the application of methods to measure muscle mass in children are one reason for this lack of data. Among modern methods used to measure muscle mass, imaging techniques require expensive equipment and expose children to undesirable doses of x-rays. These techniques also require that the patient remain still for a few minutes, which is difficult to obtain in young children. Segmental bioimpedance analysis, which does not rely on x-rays, also requires the patient to remain still and so far has not been applied to young children [39].
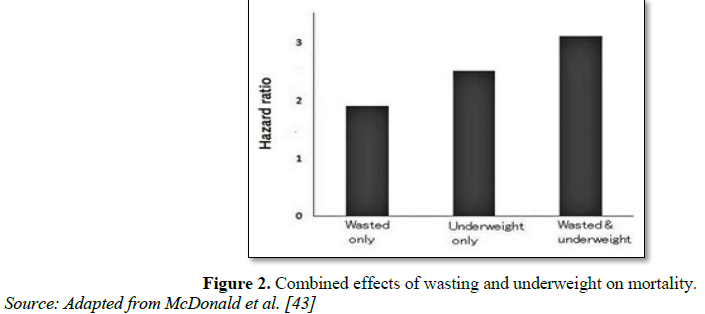
Dilution of 15N-labeled creatine, the most reliable method to measure muscle mass, has been used in only seven children with SAM compared with eight controls (often the same children) at recovery [40]. In this sample of wasted children with an average weight-for-height of 70%, the muscle mass was only 49% of the level expected for their height, showing that loss of muscle mass is disproportionately high in severely wasted children. Measurement of urinary 3-methylhistidine secretion is an indirect marker of muscle mass. Its measurement in children with SAM also suggests a drastic reduction of muscle mass in relation to body weight to less than one-third of that in well-nourished children [41]. These measurements also indicate that the relationship between creatinine and 3-methylhistidine excretion is altered in children with SAM, suggesting an abnormal muscle tissue composition. This is consistent with major histological changes in muscle tissue observed in malnourished children, with a decreased proportion of myogenic cells compared with vascular, nerve, and interstitial cells [42]. The presence of low muscle mass in wasting and the link between muscle mass and survival in a wide range of clinical conditions suggest that wasting could increase the risk of death through a decreased muscle mass [26] (Figure 1).
If malnutrition is sustained and is associated with changes in relative organ weights and reduced function of key organs, such as the heart, kidney and immune system, this can also compound the effect of the lack of fuel resulting from low muscle mass during an acute food shortage. An effect of wasting on mortality through reduced muscle mass suggests that young infants and children are especially vulnerable to malnutrition because they have a low muscle mass in relation to body weight, even in the absence of malnutrition as mentioned above [44-59].
Fat mass in wasting: Effect on mortality
Fat stores are deeply depressed in cases of wasting [60]. As noted above, in the absence of infection, fat is the main fuel for the organism in case of insufficient energy intake and survival can, therefore, be connected to fat mass [20]. Fat and especially central fat, can also play a role in maintaining the immune system, which is energy demanding when stimulated [61]. Leptin, which is produced by adipocytes and reflects body fat stores, may have a stimulating effect on the immune system by increasing cytokine and lymphocyte secretion [62,63]. Thus, fat can also be linked to survival through an effect on the immune system. In this regard, a recent study has shown that leptin levels are linked to survival in children with SAM treated in the hospital [64]. Thus, fat depletion could also provide an additional common mechanism linking wasting with increased mortality.
MULTIPLE MICRONUTRIENT INADEQUACY OF DIETS IN DEVELOPING COUNTRIES
There are several circumstances that might lead to micronutrient shortages during the lifetime of an individual living in developing countries. Such circumstances usually affect a range of micronutrients simultaneously, some of them clustering in the foods commonly consumed in those countries, i.e., iron, zinc, copper, and calcium. Hence, in populations where the major reason for iron deficiency is poor availability of iron from the diet, there is also a risk for marginal zinc status and possibly low calcium intake. In predominantly rural areas of low-income countries, the diet is based on cereal staples, that account for 60-70% of total energy intake and includes about 12% proteins, most of which are vegetable proteins. The micronutrient density of such diets is low, and the high phytate content impairs micronutrient absorption, resulting in a high risk of inadequacy. In periods of increased need, such as pregnancy, such a risk is even higher and frank deficiencies develop. Iron deficiency increases among pregnant women of most world populations. Zinc deficiency in pregnant women has been reported in Egypt [65], Nigeria [66] and Malawi [67]. Folic acid deficiency have also been described in pregnant women in South Africa [68]. Low micronutrients in mothers’ diet could potentially result in low nutrient stores at birth [69]. After birth and in the first 6 months of life, exclusive breastfeeding should allow complete coverage of energy and nutrient needs, although maternal deficiency can lead to low levels of water-soluble vitamins and possibly zinc in breast milk [70]. A comparison of predominantly breastfed babies with children given complementary foods earlier than 6 months indicates that linear growth is better in the former [71]. However, in many world populations, few children are exclusively breastfed, particularly as a result of the early introduction of liquids or other foods. According to the 4th Report on the World Nutrition Situation [72], exclusive breastfeeding rates in many countries of Sub-Saharan Africa can be as low as 1-3% and only two of the surveys reported found rates greater than 50%. Breast milk is usually replaced with fruit juice or other sweet drinks or cereal-based gruels, all of which are micronutrient-poor items, thus leading to a high risk of inadequacy. After the age of 6 months, complementary foods should be introduced in addition to breast milk. Nutrient density, the frequency of feeding and factors related to the palatability and ease of consumption of the foods (viscosity, flavor, variety) are all determinants of child micronutrient intake. A comparison of the diets of Peru and Mexico with the diets of US children indicates that the density of iron, zinc, and calcium in complementary foods is low and inadequate coverage of nutrient requirements is likely [73]. In order for the requirements of these ‘vulnerable nutrients’ to be met, the presence of animal foods such as beef, pork or chicken liver would be required. If foods with low iron bioavailability such as beans are used, more than two-thirds of the total dietary energy should be provided by that food, which is totally non-feasible. Where animal products are not available, the provision of fortified foods or supplements may be necessary. Mineral bioavailability can also be enhanced by reducing the food factors limiting absorption, e.g. by reducing hexa- and penta-inositol phosphate content with soaking, fermentation and germination [74]. Older children are fed the family diet based on cereals and pulses, in which again animal products are only occasionally present, and hence again, zinc, iron, and calcium intakes are likely to be inadequate. Data from the Nutrition CRSP supports the existence of multiple micronutrient deficiencies in developing countries. The Nutrition CRSP was a longitudinal study of the impact of marginal malnutrition on the function of infants, pre-school children, school children and adults in Mexico, Kenya and Egypt [75-77]. Multiple food intake measures on these individuals made it possible to explore the relationships between the intake of specific foods, nutrients, and growth. Table 1 presents an elaboration of the data reported in the literature for pre-schoolers, comparing the intake of energy, protein and some key micronutrients with the average intake desired for those micronutrients in that age group. Since raw data have not been used, a proper calculation of dietary adequacy cannot be done, but an overall understanding can be obtained of the nutritional value of such diets. Cereals (maize, wheat, rice) provided, respectively, 62, 68 and 71% of total energy intake; dairy products, meat, and eggs were eaten in very small quantities in all three locations, especially in Mexico and Kenya. All micronutrients were below the desired intake in Mexico and Kenya, and they were marginally sufficient for iron and copper in Egypt [78-82].
Micronutrients were also obtained largely from cereals, which are sources with low bioavailability. It is therefore easy to see how the lack of sufficient micronutrient intakes fails to sustain increased growth rates for catch-up towards the normal growth curve. This concept of micronutrient clustering in foods is well known to scientists who try to selectively induce deficiencies of individual nutrients, particularly zinc: when trying to induce zinc deficiency, copper deficiency is also induced [83-85].
HEALTH CONSEQUENCES OF WASTING
Wasting is the result of repeated insults to the growth plate, with reduced chondrocyte proliferation and maturation. A wasted child will have a lower weight than her/his peers and will resemble a younger child, usually 2-3 years younger. Wasting is also associated with a developmental delay, with the retarded achievement of the main child development milestones, such as walking. This might create an overall comparative disadvantage in an already difficult environment [86-89].
Body fat plays a critical role in regulating bone mass and linear growth [90]. The fact that wasting is a reflection of depletion in fat and muscle masses implies that a child who is wasted may suffer from linear growth. It has been demonstrated that fat tissues produce leptin, which has an influence on bone density and catch-up growth [91,92]. Fat secretes multiple hormones, including leptin, which may have a stimulating effect on the immune system. Once leptin has an effect on bone growth, it is presupposed that wasting indirectly affects linear growth. This perhaps explains why wasted children who usually have low-fat stores have reduced linear growth [93]. We do know that high fat concentration is associated with obesity. Obviously, nutrients such as sulphur, phosphorus, calcium, magnesium, vitamins D, K and C, and copper are required for skeletal growth rather than for growth of other lean tissues [94].
As previously stated, a malnourished or lower weighing child is going to be an adult of having lower weight A lower weighing adult has some functional limitations compared to a higher one, such as reduced working capacity. In societies where manpower is essential for subsistence this may have further consequences on the health and well-being not only of the individual but also of his/her dependants [69]. Wasted individuals often remain in a state of poverty throughout their lives, as they are not able to produce the extra income that might allow them to escape the cycle of mere subsistence. Reproductive performance may also be affected by stature: a lower weighing woman will usually deliver a low birth weight baby.
The combined presence of growth retardation, developmental delay, defects in cognitive function, defects in substrate metabolism, increased morbidity and mortality indicates that wasting is by no means a condition affecting just the skeletal system, although the most apparent and easily diagnosable feature is thinness. In order to advocate for a wider understanding of the problem, we would like to propose the denomination of ‘wasting syndrome’, since a syndrome is a disease entity with multiple systemic features that are sometimes maintained throughout life. Wasting cannot be considered a form of cost-free adaptation or just an indicator of socio-economic status. Thinness is not ‘beautiful’ and a lower weighing child is by no means a healthy child, nor is going to be a fit and healthy adult.
Are the features of the wasting syndrome just a coincidence or is there a common basis for their origin? We have discussed that wasting might result from past exposure to multiple micronutrient deficiencies that may still be present. In most cases, the micronutrient status of wasted children has not been investigated, both because of the technical difficulties and because of the failure to identify wasting as an active condition of poor health. Poor zinc status would compromise immunity and neurological function; iron and copper deficiency would produce anemia and affect the development of cognitive function and inadequate vitamin A status would also lead to increased susceptibility to infections. In other words, the outcome typical of the wasting syndrome, i.e. retarded growth, developmental delays, poor cognitive function, increased morbidity, and mortality could be caused by the poor status of such micronutrients.
THE PREVENTION AND TREATMENT OF THE WASTING SYNDROME
Improved methods and linkages for identification and treatment of wasting are needed, both within the health sector and cross-sectorally, in order to reduce and maintain reductions in wasting in the long-term. The global extent and consequences of wasting, particularly in some high-burden countries, has been recognized through joint statements issued by the United Nations (UN), in which the UN has endorsed community-based approaches for improving coverage of the treatment of wasting. This includes the use of MUAC as an alternative to assessing weight-for-height to aid in the timely identification of wasting.81 Additionally, decentralized outpatient treatment services are also recommended for those with severe acute malnutrition (severe wasting and/or low MUAC and/or bilateral oedema), based on community identification and referral of cases, with inpatient care also provided for those with poor appetite, severe bilateral oedema, and/or additional medical complications. Supplementary foods are provided to those who are moderately wasted and who do not have access to diets that cover their nutrient needs while their medical conditions are treated. Treatment for severe wasting is not only vital but also cost-effective, with an estimated cost of US$ 200 to treat each severely wasted child [82]. The 2013 Lancet series on under nutrition recognized treatment of severe acute malnutrition as the most cost-effective of the various direct nutrition interventions [83]. The earlier the child receives treatment, the cheaper it will be, as they are less likely to have developed additional medical complications and recovery times will be shorter. Nutrition offers one of the best returns on investment. Every US$ 1 invested in nutrition, including the treatment of severe acute malnutrition, generates as much as US$ 138 in better health and increased productivity. At the other end of the scale – not investing in nutrition perpetuates economic losses both to individuals and to countries – at an estimated cost of up to 11% of annual gross domestic product in lost productivity [84,85]. While the treatment of severe wasting is a well-established, evidence-based intervention [84,85] integrating it into essential health packages at national level has proven to be challenging. This is partly due to existing weaknesses in health systems and challenges in securing sufficient long-term funding to adequately scale up the service to the national level, as well as issues related to the supply chain and availability of treatment commodities. Moreover, the challenges in identification and treatment of wasting are also partly due to disagreements over where responsibilities lie. The international community has often supported the treatment of wasting during emergency situations. However, in order to reach the majority of children suffering from wasting in high-burden contexts, it is vital for wasting treatment to be integrated into a country’s essential health package, and for routine training and supervision of health staff involved in treatment for wasting, community mobilization and early identification, to be included as part of the curriculum [86].
In many countries where the burden of wasting is high, there are no specific activities for either treatment or prevention of moderate wasting. To manage moderate wasting in children aged 0-24 months, the package of “essential nutrition actions” should be implemented [87], including activities such as the promotion of and support for breastfeeding, nutrition counseling for families regarding complementary feeding practices and the provision of food supplements. For older children, the focus should be on improving family foods (diversity, quality, and safety). Linear programming (e.g. Optifood) is a tool that can be used to assess whether specific available foods: (i) can meet recommendations for nutrient intake; (ii) can be afforded by households; and (iii) are part of the current diet. Moderately wasted children also need to have access to health services and be treated for any medical conditions they might have. In emergency contexts, including food-insecure settings, treatment of moderate wasting usually consists of provisions of supplementary food. Beyond nutrition counseling or the increased availability of appropriate supplementary foods, the provision of cash vouchers/transfers is being explored further by a number of actors and may present advantages over product-based strategies for addressing moderate wasting. However, there is still a limited consensus among the international community about the best approaches for either the treatment or prevention of moderate wasting.
The nature of nutrition is that it spans many sectors and relationships are key to reaching multiple global targets. Currently, evidence regarding the best ways to integrate nutrition within other sectors to achieve the desired improvements is limited. The impact of nutrition-sensitive interventions on wasting (e.g. agriculture, social protection, education, water, and sanitation, etc.) has not been estimated. Improvements in the design of nutrition-sensitive services will increase the ability to:
• Identify which of these indirect programmes have the greatest effect on improving nutrition outcomes; and
• Attribute any improvement in nutritional status to the investments made.
Progress to achieve this target will depend not only on the scale-up of interventions to treat severe wasting but also on the strength and effectiveness of prevention strategies. While Ethiopia is having impressive success in treating hundreds of thousands of children each year, the large numbers of children becoming wasted are only slowly reducing, and seasonal surges of wasting are still occurring, even in years of good harvest. Better links with preventive services are urgently required in order to reduce the number of wasted children. Services should be tailored to the context and encompass a range of different services; for example, promotion of improved infant and young child feeding; promotion of good hygiene and sanitation; and better social protection policies and programmes (e.g. targeted to the poorest families who need social support to ensure access to diets that cover nutritional needs year round). Country-level contextualization is essential since strategies that are successful in Asia might not have the same success in Africa, for example. Because India accounts for approximately one half of the global burden of wasting [88], reductions in the overall burden of wasting will be highly dependent on the extent to which India places treatment and prevention of wasting as a national priority.
Finally, programmes, policy, research and financing for wasting have been separate. Wasting (and micronutrient deficiencies) shares causal pathways, which suggest that action on one is very likely to impact the other [89]. For this reason, it is important to include treatment and prevention of wasting in development plans and goals. Wasting is a condition that millions of children develop each year, with a large burden of these numbers occurring in “non-emergency” situations. It is vital that policymakers understand the importance of the problem of wasting, not only from the humanitarian perspective but with a wider lens, if the dramatic and consistent reductions in wasting are going to be achieved.
CONCLUSION
Good nutrition and a healthy lifestyle are essential throughout the whole life cycle to ensure optimal health both of the individual and future offspring. When a child misses these and his mother loves attention, he may develop wasting. Wasting is considered an indicator of poverty and this reflects the fact that in poor families the quality of diet is poor, the environment is challenging, health care is less accessible and psychosocial stimulation and parents’ care are not provided. However, wasting is not just thinness but is a truly invalidating clinical condition. Wasting is the most evident manifestation of a complex syndrome also involving reduced immune function, retarded development and impairment of cognitive function, as well as other metabolic disturbances that might affect the individual either immediately or in the long term. The wasting syndrome is one of the main forms of the widespread shortage of micronutrients in the diet, the so-called ‘hidden hunger’. Elimination and control of hidden hunger and its health-related consequences is an important public health priority that can only be achieved by ensuring adequate micronutrient intakes in all population groups. Overall improvement of the living standards will be needed and dietary diversification should be pursued in the long term. Immediate actions might involve intensive promotion of exclusive breastfeeding up to 6 months, as well as a timely introduction of complementary foods and, in situations of forcedly poor dietary patterns, food fortification or even the distribution of pharmaceutical preparations of micronutrients. In practice, a phased combination of the different types of actions should be designed. Interventions to correct growth and development are possible at least until the age of 5 years and are justified, although the extent to which catch-up is possible and the long-term implication of this remain to be clarified.
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
SOURCES OF FINANCIAL SUPPORT
There is no funding to be disclosed.
AUTHOR’S CONTRIBUTION
Mohammad Hasan Chowdhury carried out the studies, participated in the
sequence alignment, performed in the analysis of the findings and took the lead
in writing the manuscript. Lincon Chandra Shill, Nafisa Habib Purba, Farhan
Ahmed Rabbi and Md. Jayed Chowdhury contributed to the design of the study and
helped to draft the manuscript. All authors read and approved the final
manuscript.
1. UNICEF (2008) Indicators for assessing infant and young child feeding practices. Part 1: Definitions. Geneva: WHO. Available at: http://whqlibdoc.who.int/publications/2008/9789241596664_eng.pdf
2. Lomborg B (2004) Global crises, global solutions. Cambridge University Press. Available at: https://doi.org/10.1017/CBO9780511492624
3. Maluccio J, Adato M, Flores R, Roopnaraine T (2005) Breaking the cycle of poverty: Nicaraguan Red de Protección Social. International Food Policy Research Institute. Available at: http://www.ifpri.org/publication/nicaragua-red-de-protecci%C3%B3n-social-%E2%80%94-mi-familia
4. Glewwe P, Miguel EA (2007) The impact of child health and nutrition on education in less developed countries. HandbDev Econ 4: 3561-3606.
5. Bloss E, Wainaina F, Bailey RC (2004) Prevalence and predictors of underweight, stunting and wasting among children aged 5 and under in western Kenya. J Trop Pediatr 50: 260-270.
6. Bangladesh DHS (2007) National Institute of Population Research and Training (NIPORT), Mitra and Associates and Macro International. Calverton, Maryland: Bangladesh Demographic and Health Survey. Available at: https://www.unicef.org/bangladesh/BDHS2007_Final.pdf
7. Collins S, Dent N, Binns P, Bahwere P, Sadler K, et al. (2006) Management of severe acute malnutrition in children. Lancet 368: 1992-2000.
8. WHO (2010) Nutrition Landscape Information System (NLIS) Country Profile Indicators: Interpretation Guide. World Health Organization: Geneva, Switzerland. Available at: http://apps.who.int/iris/handle/10665/44397
9. Saaka M, Iddrisu M (2014) Patterns and determinants of essential new-born care practices in rural areas of northern Ghana. Int J Popul Res 2014: 1-10.
10. Legesse M, Demena M, Mesfin F, Haile D (2014) Prelacteal feeding practices and associated factors among mothers of children aged less than 24 months in Raya Kobo district, north eastern Ethiopia: A cross-sectional study. Int Breastfeeding J 9.
11. UNICEF (2012) Levels and trends in child mortality report. United Nations Children’s Fund: New York, NY, USA. Available at: http://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2012/en/
12. Bank U (2012) Levels and trends in child malnutrition: UNICEF-WHO-the World Bank joint child malnutrition estimates. Washington: Bank U. Available at: https://www.popline.org/node/552837
13. Black RE, Allen LH, Bhutta ZA, LE C e, de Onis M, et al. (2009) Action against hunger: Acute malnutrition: A preventable pandemic. International Network 247 West 37th Street, Floor 10, New York, NY 10018 212–967–7800. Available at: https://www.actionagainsthunger.org/sites/default/files/publications/Acute_Malnutrition_A_Preventable_Pandemic_01.2009.pdf
14. Van de Poel E, Hosseinpoor AR, Speybroeck N, Van Ourti T, Vega J (2008) Socioeconomic inequality in malnutrition in developing countries. Bull World Health Organ 86: 282-291.
15. Vitolo MR, Gama CM, Bortolini GA, Campagnolo PD, Drachler ML (2008) Some risk factors associated with overweight, stunting and wasting among children under 5 years old. J Pediatr 84: 251-257.
16. Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, et al. (2008) What works? Interventions for maternal and child under nutrition and survival. Lancet 371: 417-440.
17. ACC/SCN (United Nations Administrative Committee on Coordination/Sub-Committee on Nutrition) (2000) Fourth Report on the World Nutrition Situation: Nutrition throughout the life cycle. Available at: https://www.unscn.org/web/archives_resources/files/rwns4.pdf
18. United Nations Children’s Fund, World Health Organization, The World Bank (2012) UNICEF-WHO-World Bank Joint Child Malnutrition Estimates. UNICEF, New York; WHO, Geneva; The World Bank, Washington, DC. Available at: http://www.who.int/entity/nutgrowthdb/jme_unicef_who_wb.pdf
19. (2012) Levels and trends in child malnutrition: UNICEF-WHO- The World Bank Joint Child malnutrition Estimates” Available online at: http://www.who.int/entity/nutgrowthdb/jme_unicef_who_wb.pdf
20. Cahill GF Jr (2006) Fuel metabolism in starvation. Annu Rev Nutr 26: 1-22.
21. Desai M, Crowther NJ, Lucas A, Hales CN (1996) Organ selective growth in the offspring of protein-restricted mothers. Br J Nutr 76: 591-603.
22. Reeds PJ, Fjeld CR, Jahoor F (1994) Do the differences between the amino acid compositions of acute-phase and muscle proteins have a bearing on nitrogen loss in traumatic states? J Nutr 124: 906-910.
23. Lecker SH, Solomon V, Mitch WE, Goldberg AL (1999) Muscle protein breakdown and the critical role of the ubiquitin proteasome pathway in normal and disease states. J Nutr 129: 227S-237S.
24. Fernández-Real JM, Ricart W (1999) Insulin resistance and inflammation in an evolutionary perspective: The contribution of cytokine genotype/phenotype to thriftiness. Diabetologia 42: 1367-1374.
25. Caulfield LE, de Onis M, Blössner M, Black RE (2004) Under nutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria and measles. Am J Clin Nutr 80: 193-198.
26. Olofin I, McDonald CM, Ezzati M, Flaxman S, Black RE, et al. (2013) Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: A pooled analysis of ten prospective studies. PloS One 8: e64636.
27. Tanner JM (1963) Regulation of growth in size in mammals. Nature 199: 845-850.
28. Finkielstain GP, Lui JC, Baron J (2013) Catch-up growth: Cellular and molecular mechanisms. World Rev Nutr Diet 106: 100-104.
29. Wolfe RR (2006) The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475-482.
30. Tandon P, Ney M, Irwin I, Ma MM, Gramlich L, et al. (2012) Severe muscle depletion in patients on the liver transplant wait list: Its prevalence and independent prognostic value. Liver Transpl 18: 1209-1216.
31. Montano-Loza AJ, Meza-Junco J, Prado CMM, Lieffers JR, Baracos VE, et al. (2012) Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol 10: 166-173.
32. Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB (2014) Obesity paradox in cancer: New insights provided by body composition. Am J Clin Nutr 99: 999-1005.
33. Heymsfield SB, McManus C, Stevens V, Smith J (1982) Muscle mass: Reliable indicator of protein-energy malnutrition severity and outcome. Am J Clin Nutr 35: 1192-1199.
34. Marquis K, Debigaré R, Lacasse Y, LeBlanc P, Jobin J, et al. (2002) Mid-thigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 166: 809-813.
35. Soler-Cataluña JJ, Sánchez-Sánchez L, Martínez-García MA, Sánchez PR, Salcedo E, et al. (2005) Mid-arm muscle area is a better predictor of mortality than body mass index in COPD. Chest 128: 2108-2115.
36. Food and Agriculture Organization/World Health Organization/United Nations University (1985) Energy and protein requirements. Report of a joint FAO/WHO/UNU Expert Consultation. World Health Organ Tech Rep Ser 724: 1-206. Available at: http://apps.who.int/iris/handle/10665/39527
37. Briend A, Garenne M, Maire B, Fontaine O, Dieng K (1989) Nutritional status, age and survival: The muscle mass hypothesis. Eur J Clin Nutr 43: 715-726.
38. Van den Broeck J, Eeckels R, Hokken-Koelega A (1998) Fatness and muscularity as risk indicators of child mortality in rural Congo. Int J Epidemiol 27: 840-844.
39. McCarthy HD, Samani-Radia D, Jebb SA, Prentice AM (2014) Skeletal muscle mass reference curves for children and adolescents. Pediatr Obes 9: 249-259.
40. Reeds PJ, Jackson AA, Picou D, Poulter N (1978) Muscle mass and composition in malnourished infants and children and changes seen after recovery. Pediatr Res 12: 613-618.
41. Nagabhushan VS, Narasinga Rao BS (1978) Studies on 3-methyl histidine metabolism in children with protein energy malnutrition. Am J Clin Nutr 31: 1322-1327.
42. Hansen-Smith FM, Picou D, Golden MN (1978) Quantitative analysis of nuclear population in muscle from malnourished and recovered children. Pediatr Res 12: 167-170.
43. McDonald CM, Olofin I, Flaxman S, Fawzi WW, Spiegelman D, et al. (2013) The effect of multiple anthropometric deficits on child mortality: Meta-analysis of individual data in 10 prospective studies from developing countries. Am J Clin Nutr 97: 896-901.
44. Waterlow JC (1972) Classification and definition of protein calorie malnutrition. Br Med J 3: 566-569.
45. Kirksey A, Wachs TD, Yunis F, Srinath U, Rahmanifar A, et al. (1994) Relation of maternal zinc nutrition to pregnancy outcome and infant development in an Egyptian village. Am J Clin Nutr 60: 782-792.
46. Mbofung CM, Atinmo T (1987) Trace element nutriture of Nigerians. World Rev Nutr Diet. Basel, Karger 51: 105-139.
47. Huddle JM, Gibson RS, Cullinan TR (1998) Is zinc a limiting nutrient in the diets of rural pregnant Malawian women? Br J Nutr 79: 257-265.
48. Fleming AF (1996) Haematological diseases in the tropics. In: Cook C (ed): Manson’s Tropical Diseases, ed 20. London, Saunders, pp: 101-173.
49. Haram K, Nilsen ST, Ulvik RJ (2001) Iron supplementation in pregnancy - Evidence and controversies. Acta Obstet Gynecol Scand 80: 683-688.
50. (1991) Nutrition during lactation. The Institute of Medicine. Available at: https://www.nap.edu/catalog/1577/nutrition-during-lactation
51. Simondon KB, Simondon F (1997) Age at introduction of complementary food and physical growth from 2 to 9 months in rural Senegal. Eur J Clin Nutr 51: 703-707.
52. WHO (1998) Complementary feeding of young children in developing countries: A review of current scientific knowledge. Geneva, WHO. Available at: http://www.who.int/nutrition/publications/infantfeeding/WHO_NUT_98.1/en/
53. Gibson RC, Hotz C (2001) Dietary diversification/modification strategies to enhance micronutrient content and bioavailability of diets in developing countries. Br J Nutr 85: S159- S166.
54. Allen LH, Backstrand JR, Stanek EJ, Pelto GH, Chavez A, et al. (1992) The interactive effects of dietary quality on the growth and attained size of young Mexican children. Am J Clin Nutr 56: 353-364.
55. Neumann C, McDonald MA, Sigman M, Bwibo N (1992) Medical illness in school-age Kenyans in relation to nutrition, cognition and playground behaviors. J Dev Behav Pediatr 13: 392-398.
56. Murphy SP, Beaton GH, Calloway DH (1992) Estimated mineral intakes of toddlers: Predicted prevalence of inadequacy in village populations in Egypt, Kenya and Mexico. Am J Clin Nutr 56: 565-572.
57. Allen LH (1995) Malnutrition and human function: A comparison of conclusions from the INCAP and Nutrition CRSP Studies. J Nutr 125: S1119-S1126.
58. Food Composition and Nutrition Tables 1986/87: https://doi.org/10.1002/star.19870390513
59. UNICEF/WHO (2000) Feeding and nutrition of infants and young children. Geneva, UNICEF/WHO. Available at: http://www.euro.who.int/__data/assets/pdf_file/0004/98302/WS_115_2000FE.pdf
60. Waterlow JC (1992) Protein energy malnutrition. London: Edward Arnold. Available at: https://www.cabdirect.org/cabdirect/abstract/19941404451
61. Wells JCK, Cortina-Borja M (2013) Different associations of subscapular and triceps skinfold thicknesses with pathogen load: an ecogeographical analysis. Am J Hum Biol 25: 594-605.
62. Fernández-Riejos P, Najib S, Santos-Alvarez J, MartínRomero C, Pérez-Pérez A, et al. (2010) Role of leptin in the activation of immune cells. Mediators Inflamm 2010:568343.
63. Martín-Romero C, Santos-Alvarez J, Goberna R, Sánchez-Margalet V (2000) Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol 199: 15-24.
64. Bartz S, Mody A, Hornik C, Bain J, Muehlbauer M, et al. (2014) Severe acute malnutrition in childhood: Hormonal and metabolic status at presentation, response to treatment and predictors of mortality. J Clin Endocrinol Metab 99: 2128-2137.
65. Tomkins A (2000) Malnutrition, morbidity and mortality in children and their mothers. Proc Nutr Soc 59: 135-146.
66. Pelletier DL (1994) The relationship between child anthropometry and mortality in developing countries: Implications for policy, programs and future research. J Nutr 124: S2047-S2081.
67. Hoffman DJ, Sawaya AL, Verreschi I, Tucker KL, Roberts SB (2000) Why are nutritionally stunted children at increased risk of obesity? Studies of metabolic rate and fat oxidation in shanty town children from Sao Paulo, Brazil. Am J Clin Nutr 72: 702-707.
68. Gaskin PS, Walker SP, Forrester TE, Grantham-McGregor SM (2000) Early linear growth retardation and later blood pressure. Eur J Clin Nutr 54: 563-567.
69. Spurr GB (2012) The effects of chronic energy deficiency on stature, work capacity and productivity. In: Schurch B, Scrimshaw NS (eds): Effects of Chronic Energy Deficiency on Stature, Work Capacity and Productivity. Lausanne, IDECG, pp: 95-134.Available at: http://archive.unu.edu/unupress/food2/UID08E/UID08E0E.HTM
70. Kramer MS (1998) Socioeconomic determinants of intrauterine growth retardation. Eur J Clin Nutr 52: S29-S32.
71. Gross R, Schell B, Molina MC, Leao MA, Strack U (1989) The impact of improvement of water supply and sanitation facilities on diarrhea and intestinal parasites: A Brazilian experience with children in two low-income urban communities. Rev Saude Publ 23: 214-220.
72. Ramakrishnan U, Manjrekar R, Rivera J, Gonza´les-Cossı´o T, Martorell R (1999) Micronutrients and pregnancy outcome: A review of the literature. Nutr Res 19: 103-159.
73. Lwambo NJ, Brooker S, Siza JE, Bundy DA, Guyatt H (2000) Age patterns in stunting and anaemia in African school children: A cross-sectional study in Tanzania. Eur J Clin Nutr 54: 36-40.
74. Habicht JP, Martorell R, Rivera JA (1995) Nutritional impact of supplementation in the INCAP longitudinal study: Analytic strategies and inferences. J Nutr 25: S1042-S1050.
75. Rivera JA, Gonzalez-Cossio T, Flores M, Romero M, Rivera M, et al. (2001) Multiple micronutrient supplementation increases the growth of Mexican infants. Am J Clin Nutr 74: 657-663.
76. Branca F, Lopriore C, Guidoum Y, Briend A, Golden MH (1999) Multi-micronutrient fortified food reverses growth failure and anaemia in 2-5 year old stunted refugee children. Scand J Nutr 43: S51.
77. Grantham-McGregor S, Walker S, Powell C (1991) Nutritional supplementation and mental development. Lancet 338: 758.
78. Cianfarani S, Geremia C, Germani D, Scire G, Maiorana A, et al. (2001) Insulin resistance and insulin-like growth factors in children with intrauterine growth retardation. Is catch-up growth a risk factor? Horm Res 22: S7-S10.
79. Allen LH (1995) Malnutrition and human function: A comparison of conclusions from the INCAP and nutrition CRSP studies. J Nutr 125: S1119-S1126.
80. Food Composition and Nutrition Tables 1986/87: Souci/Fachmann/Kraut. https://doi.org/10.1002/food.19870310519
81. World Health Organization (2012) Global database on child growth and malnutrition. Joint child malnutrition estimates – Levels and trends. UNICEF-WHO-The World Bank project. Geneva: World Health Organization. Available at: http://www.who.int/nutgrowthdb/estimates2012/en/
82. Horton S, Shekar M, McDonald C, Mahal A, Brooks JK (2010) Scaling up nutrition. What will it cost? Washington DC: The World Bank. Available at: https://openknowledge.worldbank.org/handle/10986/2685
83. Dadhich JP, Faridi MMA (2013) Maternal and child nutrition. Lancet 382: 1549.
84. Black RE, Cousens S, Johnson HL, Lawn JE, Rudan I, et al. (2010) Global, regional and national causes of child mortality in 2008: A systematic analysis. Lancet 375: 1969-1987.
85. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, et al. (2013) Maternal and child under nutrition and overweight in low-income and middle-income countries. Lancet 382: 427-451.
86. Dolan C, Khara T, Acosta A, Shoham J (2012) Government experiences of scale up of Community-based Management of Acute Malnutrition (CMAM): A synthesis of lessons. Oxford: Emergency Nutrition Network. Available at: https://www.ennonline.net/cmamgovernmentlessons
87. (2013) Essential nutrition actions: Improving maternal, new-born, infant and young child health and nutrition. Geneva: World Health Organization. Available at: https://www.ncbi.nlm.nih.gov/books/NBK258736/
88. UNICEF, WHO, World Bank, UN-DESA Population Division (2013) Levels and trends in child mortality 2013. Geneva: World Health Organization. Available at: http://www.who.int/maternal_child_adolescent/documents/levels_trends_child_mortality_2013/en/
89. Khara T, Dolan C (2014) Technical briefing paper: Associations between wasting and stunting, policy, programming and research implications. Oxford: Emergency Nutrition Network. Available at: http://files.ennonline.net/attachments/1862/WAST_140714.pdf
90. Dewey KG, Hawck MG, Brown KH, Lartey A, Cohen RJ, et al. (2005) Infant weight-for-length is positively associated with subsequent linear growth across four different populations. Matern Child Nutr 1: 11-20.
91. Karsenty G (2006) Convergence between bone and energy homeostases: Leptin regulation of bone mass. Cell Metab 4: 341-348.
92. Gat-Yablonski G, Ben-Ari T, Shtaif B, Potievsky O, Moran O, et al. (2004) Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology 145: 343-350.
93. Popkin BM, Richards MK, Montiero CA (1996) Stunting is associated with overweight in children of four nations that are undergoing the nutrition transition. J Nutr 126: 3009-3016.
94. Golden MH (2009) Proposed recommended nutrient densities for moderately malnourished children. Food Nutr Bull 30: S267-S342.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Astronomy and Space Research
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)