1754
Views & Citations754
Likes & Shares
Graft-versus-host
disease (GVHD) is a significant cause of non-relapse mortality after allogeneic
hematopoietic cell transplantation (allo-HCT). Existing strategies to prevent
and treat GVHD are incomplete, where a significant portion of allo-HCT
recipients developed this complication. Despite this, one such therapy has
emerged involving the use of regulatory T cells (Tregs) to control GVHD. The
use of natural Tregs (nTregs) yielded positive pre-clinical results and are
actively under investigation to reduce GVHD. However, broad application of this
approach may require standardization of Treg expansion methods and dosing.
Inducible Tregs (iTregs) can be seamlessly generated, but controversial
pre-clinical findings and phenotype instability have hampered their translation
into the clinic. Here, we review the current biological differences between
nTregs and iTregs, as well as their effects on GVHD and graft-versus-leukemia
(GVL) responses. We conclude by exploring the idea of combinational cellular
therapies for the prevention of GVHD and preservation of GVL.
Abbreviations: allo-HCT: Allogeneic Hematopoietic Cell Transplantation; GVHD: Graft-Versus-Host Disease; GVL: Graft-Versus-Leukemia; Tregs : Regulatory T Cells; Ntregs : Natural T Regulatory Cells; Itregs: Inducible T Regulatory Cells; Teffs : Effector T Cells; HLA: Human Leukocyte Antigen; Apcs : Antigen Presenting Cells; IPEX: Immunedysregulation Polyendocrinopathy Enteropathy X-Linked Syndrome; MHC: Major Histocompatibility Complex; MDSC: Myeloid Derived Suppressor Cells; RA: Retinoic Acid; TCR: T Cell Receptor; CTLA-4: Cytotoxic T-Lymphocyte-Associated Protein 4; LAG-3: Lymphocyte Activation Gene 3; ATP : Adenosine Triphosphate; DC: Dendritic Cells; CNS2: Conserved Non-Coding Sequence 2; TSDR: Treg Specific Demethylation Region; CDK2: Cyclin-Dependent Kinase 2; Ezh2: Chromatin-Modifying Enzyme; PTEN : Phosphatase and Tensin Homolog, LCL: Lymphoblastoid Cell Line; Mihags : Minor Mismatch Antigens; Damps : Danger-Associated Molecular Pattern.
Keywords: Graft-versus-host disease (GVHD), nTregs, iTregs, Cellular therapy.
INTRODUCTION
Allogeneic
hematopoietic cell transplantation (allo-HCT) provides a reconstituted, healthy
immune system for patients suffering from bone marrow failure syndromes and
hematological malignancies such as leukemias, lymphomas, and myelomas. Donors
are identified by high-resolution typing of class I and II human leukocyte
antigen (HLA), and typically selected by recipient matching at HLA-A, -B, -C,
-DRB1, DQB1, and –DPB1 [1].
Disparity within the major HLA, or even minor histocompatibility antigens [2],
may stimulate donor T cells to induce GVHD. However, this is offset by the
anti-cancer graft-versus-leukemia (GVL) effect of the allograft.
The
pathophysiology of GVHD is complex, involving many different T-helper cell
types which contribute to disease manifestation; we refer the readers to our
extensive review discussing the characteristics of these cells [3].
In brief, following
conditioning, damage to host tissues causes the release of pro-inflammatory
cytokines and danger-associated molecular pattern molecules (DAMPs), which in
turn activate recipient antigen-presenting cells (APCs).
These host APCs
then present host antigens to the donor T cells, which rapidly expand and
differentiate into effector T cells (Teffs). Following differentiation, Teffs
migrate to the GVHD target organs (skin, liver, lung, and gut) and cause end
organ damage [3]. Despite extensive advancements in HLA matching,
immunosuppressive drugs, and conditioning therapies, many patients that receive
allo-HCT still succumb to primary disease (37%), GVHD (20%), or infection
(17%), respectively [4]. Clearly, there is room for improving the success of
allo-HCT. Many clinicians and scientists have begun to embrace the concept of
harnessing our own suppressive immune cells, T regulatory cells (Tregs), to
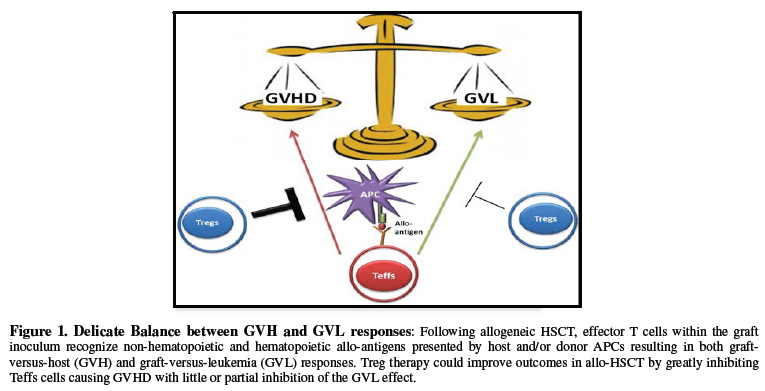
improve recipient survival and quality of life [5-7]. A delicate balance exists
between GVL and GVHD responses, with too much suppression leading to tumor
relapse and too little suppression leading to alloreactivity and end organ
damage (Figure 1). Alas, balancing these fine cellular mechanisms has
yet to be realized. Nonetheless, Tregs, with their ability to acquire antigen
specificity, may be the answer clinicians and scientists have been looking for.
Tregs are
relatively young, first being described as “suppressor T cells” in the 1970’s
by Gershon and Kondo, who conducted elegant experiments illustrating that
induction of tolerance was dependent on thymus-derived lymphocytes, and not B
cells [8,9]. However, due to the inability to clearly characterize this
suppressive lymphocyte population, controversial findings within the I-J region
[10], and
limitations in scientific techniques, the “suppressor T cells” fell off the
scientific map for 12 years. In 1982, Sakaguchi and colleagues, while studying
the effects of neonatal thymectomy on normal immune homeostasis, stumbled upon
a very important discovery: within the CD4 T lymphocyte compartment were cells
capable of causing autoimmune disease and those capable of preventing it [11].
Thirteen years later, Sakaguchi was able to distinguish a reliable cell surface
marker (CD25) which could differentiate between the protective CD4 T cells
(CD25hi) fraction from the pathologic CD4 cells (CD25low)
[12]. However, activated T cells can also express CD25, therefore negating the
exclusivity of CD25 as marker for Tregs [13]. Luckily, advances in
intracellular staining techniques allowed for the discovery of Foxp3 (a member
of the forkhead winged helix family), the master transcription factor for
determining Treg fate and suppressive function [14]. The specificity of Foxp3
to the Treg lineage was solidified by the finding that patients suffering from
the autoimmune disease immunedysregulation polyendocrinopathy enteropathy
X-linked syndrome (IPEX) had inherited germline mutations within the FOXP3 gene,
which resulted in non-functional Tregs [15]. Scrufy mice, harboring
a deletion of the Foxp3 gene, also display a lymphoproliferative
disease characterized by multiorgan damage. The ability to definitively isolate
and study Tregs (CD4+CD25+Foxp3+) in
autoimmune diseases clearly shows that the major function of these cells is to
maintain immune homeostasis.
Characteristics
of T regulatory cells
Development and
Generation
With the identification of Foxp3, studies on Tregs
increased exponentially and soon after we would find that regulatory cells of
the immune system were not just confined to expression of Foxp3 or even the T
cell compartment. Over the years, multiple different flavors of regulatory
cells have been discovered: Tr1 cells [16], CD8+-Tregs
[17,18] myeloid derived suppressor cells (MDSC) [19], and B cells (B10 cells)
[20].
In this review, we will focus on CD4+CD25+Foxp3+ regulatory
T cells. As stated in the introduction, early neonatal thymectomy on day 3
versus day 7 of life pointed to the thymus as a major tissue associated with
generation of Treg [21]. Experiments transferring the CD25+CD4+ Tregs
from the periphery and the resulting abolition of autoimmune disease in Scurfy
mice [14] hinted that the Treg pool was actually comprised of two distinct
subsets. Indeed, it is now widely accepted that Tregs can be either naturally
derived from the thymus (nTregs) or converted from naïve CD4+CD25- T
cells in the periphery termed as inducible Tregs (iTregs).
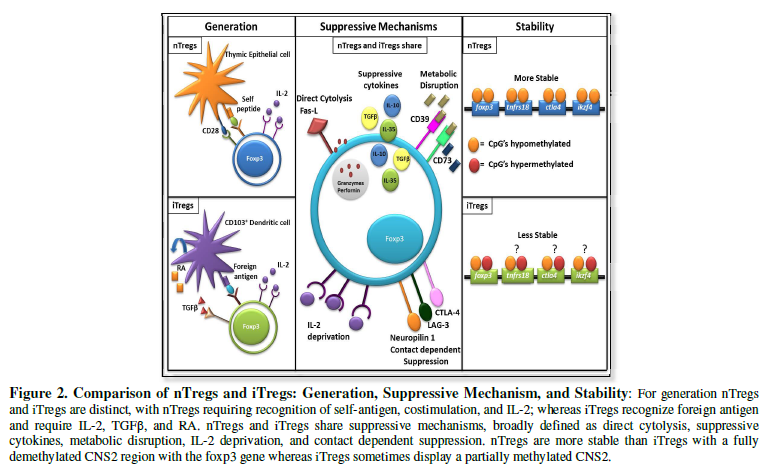
Both nTregs and
iTregs have differential requirements for their generation, which helps
characterize these two distinct subsets. nTregs are derived exclusively from
the thymus. Upon recognition of self-antigen/self-MHC (major histocompatibility
complex) with high affinity [22,23], co-stimulation from CD28/B7 interactions
[24] and IL-2 (although not required) [25], nTregs
begin to increase expression of Foxp3 and acquire suppressive function [26,27].
iTregs, on the other hand, arise in the periphery from a population of naïve T
cells, and therefore do not recognize self-antigens with high affinity [28].
Instead, during chronic antigen exposure, including microbes in the gut and
with suboptimal co-stimulation through CD28/B7, iTregs initiate the expression
of Foxp3. In contrast to nTregs, iTregs require the presence of exogenous
cytokines, IL-2 [25] and TGFβ [28], to
fully differentiate into the commonly known suppressor T cells. Retinoic acid,
(RA) produced by CD103+ dendritic cells (DC) in the gut, has
also been shown to further drive conventional T cells to express Foxp3 [29,30]
(Figure 2).
Suppressive
Mechanisms
While nTregs
and iTregs may differ in their requirements for generation, they utilize a
multitude of similar mechanisms in order to maintain immune homeostasis
[31,32] (Figure 2).
Tregs are activated via TCR engagement, which is absolutely necessary to
mediate their suppressive function in vivo. In an elegant
study using inducible genetic ablation of cell surface TCR complexes, Levine
and colleagues found that TCR stimulation was surprisingly not required for
Foxp3 expression, stability, or the ability of Tregs to consume IL-2 [33].
Instead, TCR activation is necessary for the expression of a limited number of
genes, like IRF4, that are required for activated Tregs to maintain
suppressive function [33]. The suppressive mechanisms of Tregs can be broadly
classified into contact-dependent or contact-independent suppression.
Contact-dependent suppression involves the expression of inhibitory molecules:
CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), LAG-3 (lymphocyte
activation gene 3), and Neuropilin-1. CTLA-4 inhibits expression of the
costimulatory markers CD80/CD86 on the surface of APCs through trans-endocytosis [34], and
thus results in decreased proliferation of T cells. Specific deletion of CTLA4
in Tregs resulted in decreased suppressive function [35]. LAG-3 binds to
MHC-Class II with a high affinity [31] on immature DCs and inhibits their
maturation and co-stimulatory capacity [36]. Neuropilin-1, a recently
discovered component of the Treg suppressive arsenal, was found to potentiate
long-lasting interactions between Tregs and DCs. Neuropilin-1 ablation resulted
in attenuated Treg suppressive function [37,38].
In conjunction with contact-dependent suppression,
Tregs utilize contact-independent mechanisms that create an immunosuppressive
milieu which can counteract the inflammatory milieu. A brief list of such
mechanisms include the secretion on anti-inflammatory cytokines (IL-10, TGFβ,
and IL-35), IL-2 consumption, release of granzymes, and generation of adenosine
through ectoenzymes CD39/CD73 on the Treg surface (Figure 2). IL-10, an
immunoregulatory cytokine, seems to act as a tissue- specific suppressive
mechanism utilized by Tregs at intestinal interfaces. In an induced colitis
model, IL-10 deficient Tregs could not protect mice during transfer of CD45RBhighCD4+ T
cells [39]. Likewise, Rubtsov and colleagues generated a specific IL-10
ablation within Foxp3 expressing cells and found 40% of IL-10 deficient mice
developed spontaneous colitis by 6 months of age. However, these same mice did
not develop systemic autoimmunity [40]. The major function of TGFβ-mediated
Treg suppression is surprisingly through contact-dependent, but
APC-independent, induction of infectious tolerance via a process of converting
naïve or Teffs into suppressive CD4+Foxp3+ suppressor
T cells [41]. IL-35, much like TGFβ, has been implicated in conferring
infectious tolerance by inducing iTr35 regulatory cells, which mediate
suppression via IL-35 [42].
Interestingly, high expression of CD25 (IL-2 receptor alpha chain) not
only aids in the identification of Tregs but also allows Tregs to
non-specifically sequester IL-2 from the inflammatory microenvironment. This
effect was illustrated when addition of common-γ chain cytokines reversed Treg-mediated
T-cell apoptosis in vitro and in vivo [43].
Since Tregs require activation through TCR signaling, it is no surprise that
they also express the ectoenzymes CD39/CD73, which convert extracellular
adenosine triphosphate (ATP) into adenosine [44,45]. Tregs utilize adenosine by
increasing its concentration within the inflammatory microenvironment, which
increases adenosine binding to A2A adenosine receptors expressed on DCs and T
cells. This leads to a subsequent increase of cyclic AMP, which results in inhibition
of DCs and T cells [46]. Finally, Tregs can cause direct apoptosis of Teffs
through the release of granzymes [47].
With regards to GVL/GVHD responses, the role of
granzymes generated by Tregs is complex. Ley and colleagues found that granzyme
B-expressing Tregs specifically accumulated in the tumor microenvironment and
directly caused granzyme-mediated apoptosis of NK and CD8 Teffs, inhibiting
tumor clearance. How [48] ever, some years later, Ley also noted that Tregs do
not use granzyme B to mediate apoptosis in controlling Teffs during GVHD [49].
More recently, granzyme A was shown to be critical for Tregs in controlling
intestinal GVHD. In this study, mice treated with Tregs deficient for granzyme
A failed to rescue hosts from gastrointestinal GVHD [50]. IL-10 was also found
to be a key factor utilized by nTregs to suppress GVHD, as CD4+CD25+ Tregs
from IL-10-/- mice were ineffective in alleviating acute GVHD
[51]. Homing to lymph nodes and target organs via CCR5 expression is also
indispensable for the ability of Tregs to suppress GVHD. Genetic ablation of
CCR5 negates the Treg’s ability to attenuate GVHD [52]. Another important
molecule that is required for Tregs to suppress GVHD is CD62L, when CD62L is
expressed at low levels on Tregs they cannot effectively home to the lymph
nodes and suppress early activation of Teffs [53,54]. Hence, it seems Tregs use
a vast repertoire of suppressive mechanisms to regulate immune reactions in a
context and tissue-specific manner. Further research is needed to exploit these
aspects of Treg suppression for maximal therapeutic efficacy.
Stability
In order to
effectively incorporate nTregs or iTregs as a cellular therapy, whether for
GVHD or autoimmune disorders, strict precautions must be taken to ensure patient
safety. The advantage of cellular therapy is that these Tregs arise naturally
to promote immune homeostasis. Therefore, off-target side effects, like those
seen with pharmacological therapy, should be reduced significantly. However,
two different lineage-tracing studies revealed that Foxp3 expression could be
lost in a subset of Tregs, referred to as “ex-Tregs”.
The degree of
stability varied based on the tracking system deployed by each lab, in one
study when Foxp3 was tagged using NOD BAC transgenic mice expressing GFP-Cre
within the Foxp3 promoter crossed with ROSA-LSL-YFP mice, allowing Tregs to be
labeled with YFP before loss of Foxp3-GFP, the investigators reported 10-15% of
Tregs to be GFP-YFP+ “ex-Tregs” [55].
When another group used a tamoxifen-inducible
GFP-Cre fusion with the estrogen receptor mutant (GFP-creERt2) crossed with
ROSA-LSL-YFP, allowing for transient tagging of Tregs, they reported 96% of
Tregs to be stable GFP+YFP+ even under inflammatory
conditions [56]. The discrepancy between these two lineage-tracing studies is
still under active investigation. Hesitation among clinicians and
scientists began after these initial lineage-tracing studies and was amplified
with the finding that nTregs can lose expression of Foxp3 after repeated rounds
of ex vivo stimulation [57,58]. Taking these finding into account, a major
concern becomes apparent: how can we ensure the Treg cellular therapy remains
suppressive and safe if the master transcription factor and regulator of
suppressive function, Foxp3, is lost?
The questions surrounding the environmental factors, external stimuli,
and intrinsic mechanisms that maintain or negate the expression and stability
of Foxp3 have become extraordinarily prevalent in the field of Treg research,
and still remain a hot topic of debate. Recently, numerous extensive reviews
have explored the notion of Treg stability versus Treg plasticity, with the
general consensus being that Tregs possess the ability to display both of these
characteristics depending on the microenvironmental signals they receive
[59,60]. Treg stability can be generally separated into two subsets: the epigenetic
control of Foxp3 (gene regulation) and the stability of Foxp3 (transcription
factor maintenance). Classically, a stable Treg’s genetic signature consisted
of highly demethylated CpG islands within the conserved non-coding sequence 2
(CNS2) in the Treg-specific demethylation region (TSDR), with nTregs displaying
fully demethylated CNS2 and iTregs displaying partially demethylated CNS2
regions [61]. However, the field of Treg genetic stability has moved from a
Foxp3 centric view to a multiple Treg-signature gene view, termed “nTreg-Me” by
Ohkura et al. [62]. In these experiments, it was demonstrated that CpG
hypomethylation of four Treg signature genes: Foxp3, Tnfrs18 (GITR),
Ctla4, and Ikzf4 (Eos) was independent of Foxp3 expression and
occurred following strong and/or chronic TCR signaling. Importantly, it was
found that cells expressing Foxp3, but without a full nTreg-Me signature, can
lose stability and become plastic, secreting proinflammatory cytokines (62) (Figure
2). In line with this study was the establishment of the Treg-quintet: a
complex of five redundant transcription factors that act in conjunction with
Foxp3 to fully establish the Treg-signature [63]. Any one of these factors,
Eos, IRF4, GATA-1, Lef-1, and Stab1 can help stabilize Foxp3 after it binds its
target site, resulting in either repression of IL-2 or enhancement of CTLA-4
expression, thus fully committing the cell to the Treg phenotype.
Given that
expression of the Foxp3 protein itself ensures inheritable maintenance of the
Treg phenotype through direct binding to the CNS2 in a Cbfb-Runx1 demethylation
dependent manner [61], any investigators have shifted their focus to
identifying what factors contribute to the stability of Foxp3 expression.
Recently, some key negative (CDK2 and Stub1) and positive (PTEN and Ezh2)
regulators have emerged. Cyclin-dependent kinase 2 (CDK2) was found to phosphorylate
Foxp3, which then recruits the E3 ubiquitin ligase Scf/Fwb7. Furthermore, when
CDK2 was genetically deleted, the half-life of Foxp3 was dramatically
increased, resulting in a more potently suppressive Treg [64]. Likewise, the E3
ubiquitin ligase, Stub1, was found to polyubiquinate Foxp3 in a heat shock
protein 70-dependent fashion during inflammatory responses [65]. Silencing of
Stub1 decreased the degradation of Foxp3 and enhanced protection from T cell
mediated colitis in mice [65]. Conversely, phosphatase and tensin homolog
(PTEN) deficiency lead to a loss of CD25 expression, and eventual loss of Foxp3
expression and suppressive function. This effect can likely be attributed to
overt signaling through PI3(K), a direct target of PTEN [66,67]. Finally, the
chromatin-modifying enzyme (Ezh2) was found to aid Foxp3 in binding to
repression target genes (IL-2 and IFNγ) in order to silence them. Genetic
ablation of Ezh2 lead to a decrease in Foxp3+ cells in
non-lymphoid tissues and expression of genes resembling Teffs at those sites
[68]. Hence, Ezh2 deficiency in this context failed to protect mice from
autoimmune colitis [69]. More specifically, Ezh2 may impacts Tregs in tissue
specific manner as Ezh2 deficient Tregs displayed reduced expansion on the spleen
and lymph nodes, but not in the thymus and lamina propria [69]. Furthermore, He
et al. demonstrated that Ezh2 plays an important role in Treg survival and
expansion post BMT [70]. Extensive research is needed to understand exactly
what can make, and more importantly, maintain a stable Treg phenotype if we
hope to one day apply Treg therapy in a clinical setting.
Harnessing
Tregs for Cellular Therapy in GVHD
nTregs
Given their
natural presence, high stability, and important function in maintaining
homeostasis, nTregs were the first subset of Tregs to be explored as an option
for cellular therapy. The uncontrolled immune activation, high likelihood of
disease (GVHD), limited therapeutic options, and steroid refraction that
surround allo-HCT made nTregs an ideal candidate for a potential therapeutic.
Initial experiments in pre-clinical models found that donor-type CD25+CD4+ Tregs
could suppress lethal acute GVHD in BALB/c recipients, but only if a high ratio
of 1:1 (Tregs: Teffs) was maintained [51]. The knowledge that nTregs only
account for 5-10% of the total CD4 T-cell population and that a high number was
needed to achieve GVHD attenuation made it clear that nTregs would need to be
expanded ex vivo in order to achieve a more effective therapy.
A seminal study from Blazar’s group in 2002 tested ex vivo polyclonal
activated and expanded nTregs in three different models of lethal acute GVHD
[71]. Importantly, this study established that nTregs can be expanded (67-fold)
to sufficient numbers that can attenuate GVHD, thus offering a solution to the
problem of low circulating nTregs. To further assess clinical applicability,
investigators strove to see if nTregs would suppress the beneficial GVL effect.
Using two different tumor models, A20 and BCL1, it was demonstrated that
freshly isolated CD4+CD25+ Tregs did not impair the
ability of Teffs to clear tumor at a 1:1 ratio. However, if the Teffs dose was
below a certain threshold, the tumor relapsed [72].
With the strong
preclinical findings indicating that nTregs could functionally attenuate GVHD
while maintaining GVL, the field moved quickly to translate murine findings to
human nTregs. Levings isolated CD4+CD25+ human nTregs
from peripheral blood and expanded them with IL-2 and allogeneic feeder cells.
These expanded nTregs remained unresponsive to allogeneic DCs and anti-CD3
activation, while maintaining the ability to suppress autologous CD25- T
cells in vitro [73]. nTreg expansion of 100-fold was reached
by Godfrey in 2004, using cell-sized dynabeads with anti-CD3 and anti-CD28
attached, CD4 feeder cells, and IL-2 [74]. It was found that these activated
and expanded nTregs could potently suppress DC-driven allogeneic mixed
lymphocyte reactions by 90%, and completely prevent the secretion of pro-
inflammatory cytokines [74]. Since cord blood transplants are often used in the
clinic, researchers also tested whether nTreg isolation and expansion from this
source could also be effective. Cord blood was found to contain a larger CD25bright population
compared to adult peripheral blood, in which the population was CD25dim indicating
a non-suppressive function. These nTregs displayed a comparable growth rate to
peripheral nTregs, and were also potently suppressive against allogeneic CD4+CD25- Teffs
[75]. Lastly, based on the finding that human nTregs could be expanded more
robustly using anti-CD3 loaded artificial APCs and could potently suppress
xenogeneic GVHD [76], the
first clinical trials were initiated for nTreg therapy for the treatment of
GVHD.
Recently, a new concept has emerged regarding the
expansion of nTreg cells for cellular therapy: selective expansion of the
alloreactive nTregs within an apheresis product. This more personalized
approach, using nTregs specific for both HLA-mismatched [7] and HLA-matched but
minor antigen mismatched (miHAgs) [77], yielded
a high number of potently suppressive nTregs. These results have initiated the
first clinical trial using personalized nTregs to prevent acute GVHD [6].
In 2009, the first patients were treated with ex
vivo expanded CD4+CD25+CD127- nTregs from
donor peripheral blood [78]. In this initial trial, only two patients were
enrolled, as nTreg therapy could only be initiated once standard
immunosuppression failed. One patient developed acute GVHD and displayed
transient alleviation of disease; however, the Treg source became exhausted and
the patient later succumbed to multiorgan failure [78]. The other patient
developed chronic GVHD. Yet, once nTreg therapy was initiated, a significant
reduction in symptoms was observed [78]. Even though the sample size was very
small, this study lead to the first dose escalation study for ex vivo expanded
nTregs isolated from umbilical cord blood [79]. A dosing of 1, 3, 10, or 30 x
106 Tregs/kg was tested. Of the 23 patients enrolled, 17
patients received their target dose and no dose-limited toxicities were
observed. A modest reduction in acute GVHD was observed in the 23 patients,
compared with historical controls (43% vs 61%, respectively) [79]. In a very
bold clinical trial, freshly isolated nTregs from donor peripheral blood were
administrated four days prior to transplant, followed by no post-transplant immunosuppression.
Of the 26 patients enrolled, only 2 developed GVHD. Given that no
immunosuppression was used, this trial proved that nTregs could be used as a
prophylactic for GVHD [80]. However, 13 of the 26 patients died within 3 months
post-transplant from other co-morbidities. These three clinical trials have
opened the door to a realm of possibilities for Treg therapy. However, there
are still improvements that need to be made. For instance, the expansion
potential of nTregs remains a major obstacle, as 5 patients did not receive
sufficient cell doses [79]. Also, despite the success of using freshly isolated
nTregs, a high ratio of 2:1 (Treg: Teff) was still needed to prevent GVHD [80].
iTregs
The study of iTregs in pre-clinical models of GVHD
has been restricted to in vitro generation of iTregs due to
the fact that an adequate marker to fully distinguish nTregs from iTregs has
not been established. Given that conventional T cells comprise a larger
percentage of peripheral blood or cord blood products and have an increased
activation capacity compared to nTreg cells, protocols to polarize these cells
into iTregs are currently being investigated. It is now well established that
conventional CD4 T cells isolated from peripheral lymphoid organs can begin to
express Foxp3 upon polyclonal stimulation with anti-CD3/anti-CD28 in the
presence of TGFβ and IL-2 [28,81,82]; and the addition of retinoic acid (RA)
can further enhance the expression of Foxp3 [29].
Unlike nTreg
preclinical findings, which displayed similar results even across different
expansion and GVHD models, there is still considerable controversy in the
literature regarding iTreg therapy for the prevention or treatment of GVHD.
This controversy seems to encompass differences in activation reagents,
polarizing cytokines, and infusion schedule (Table 1). iTregs generated
using polyclonal activation (anti-CD3/anti-CD28) [83,85] are inferior.
Antigen-specific
[86,87] allo-antigen specific iTregs [88,89]. Beres et al illustrated that a
high percentage of conversion can be achieved using polyclonal activation;
however, even at a 1:1 (Treg: Teff) ratio, these iTregs could not effectively
attenuate acute GVHD [83]. They claim that the ineffectiveness of iTreg therapy
directly stems from the loss of Foxp3 expression. This finding agrees with the
subsequent study by Zhang et al, which showed that polyclonal activated iTregs
failed to protect recipient mice and could even be pathogenic if systemic
rapamycin and IL-2 complexes were not co-administrated [84]. Despite these two
pre-clinical findings, Hippen et al was able to induce naïve T cells from human
peripheral blood products, and generated 240 x 109 iTregs after
stimulation with KT64/86 cells (a K562 cell-based artificial APC with
expression of CD86 and high affinity Fc receptor loaded with anti-CD3); these
iTregs potently suppressed xenogeneic GVHD [85]. Alternatively, we have shown
that using OT-II and HY-transgenic naïve T cells stimulated with either OVA
[86] and HY peptide [87], induced
a large amount of antigen-specific iTregs that potently suppress acute GVHD,
even at low Treg: Teff ratios in both cases. This higher potency is attributed
to the ability of antigen-specific iTregs to recognize antigen, as these
antigen-specific iTregs failed to protect recipient mice when the cognate
antigen was not expressed. This further emphasizes that continuous activation
of Tregs through TCR engagement is essential for their suppressive function. In
a non-irradiation BMT model, when naïve B6 T cells were used to generate
induced alloreactive iTregs with BALB/c BM-derived mature DCs [88], the
generated iTregs proved ineffective in protecting mice from GVHD. This was due
primarily to loss of Foxp3 expression. In contrast, when CD11c+ splenic
DCs [89] were used to generate induced alloreactive iTregs in the same manner,
mice had significantly attenuated GVHD, and these iTregs were able to persist
for 6 months in recipient mice. We have adapted the method established by Sela
et al. and generated alloreactive CD4 iTregs, and have found these iTregs to be
potently suppressive, and effectively attenuate GVHD in a major MHC-mismatched
irradiated BMT model (unpublished observations). It is no surprise that
antigen-specific iTregs are more potent and suppressive than polyclonal iTregs.
According to a recent study, the two different activation signals impart
different phenotypic profiles to each iTreg [90]. Physiologically activated
iTregs displayed better control of Th1 responses as well as a broader range of
chemokine and chemokine receptor expression than anti-CD3/CD28 activated iTregs
[90]. This is a potential explanation for the differences seen between
investigators with regards to the iTregs ability to attenuate GVHD.
Differences in the polarizing conditions would also
account for the discrepancy seen in iTreg therapy in controlling GVHD. IL-2 and
TGFβ are present throughout all experiments performed, however, some
investigators use rapamycin [84,85], while others use RA [83,86-89]. Since
rapamycin has been shown to preferentially suppress Teffs while allowing for
the growth/conversion of iTregs, the addition of this compound to generation
conditions should yield a more pure population of iTregs [91]. Yet, our lab and
others have proven that RA greatly increases the amount of naïve T cells
converted into iTregs, which exhibit potent suppressive function. An important
reason for this is that RA has been shown to increase the histone acetylation
and methylation within the CNS elements of the Foxp3 promoter region, thus
increasing accessibility of binding partners to the Foxp3 promoter [92].
Finally, the infusion schedule seems to play a
major part in determining the degree of GVHD attenuation using iTreg therapy.
Almost all studies use iTregs as a prophylactic therapy, as iTregs have yet to
be shown to be beneficial as a treatment modality.
Most
investigators infuse iTregs with T-cell depleted bone marrow and CD25-depleted
Teffs within 24 hours of irradiation [83,84,88,89]. Noting the observation that
initial infusion of nTregs two days prior to Teffs infusion resulted in a
robust expansion of nTregs and a 10-fold decrease in the amount of Tregs needed
to attenuate GVHD [93], we strove to apply this infusion schedule to iTreg therapy.
Indeed, we
found infusion of iTregs prior to Teffs greatly increased the potency of iTregs
in attenuating GVHD [87]. Despite these conflicting results, the first dose
escalation in a clinical trial using iTregs [85] will be tested in adults
receiving non-myeloablative HLA-identical sibling donor transplantation [94].
We are eagerly awaiting the outcome of this trial, as it will further
contribute to our understanding of iTreg cellular therapy.
Preserved or
Compromised GVL
Although
attenuation of GVHD is the main focus of investigators in assessing the
potential for Treg therapy, suppression of Teffs can only reach a certain
threshold before these cells are unable to clear recipients of residual tumor
cells (the GVL effect). In fact, the increase in Treg numbers in the peripheral
blood and/ or tumor microenvironment positively correlates with tumor relapse
or growth in mice and humans [95,96]. With regards to nTreg therapy,
pre-clinical models show contrasting results depending on the type of tumor tested.
In models using A20 [72,97] and BCL1 [72], Tregs
did not inhibit the GVL effect. However, the GVL effect was only slightly
inhibited in a model using P815 mastocytoma [97]. Be that as it may, initial
nTreg clinical trials indicated no increased incidence of tumor relapse
compared to historical controls [79]. iTregs, on the other hand, seem to be
more complex. Zhang et al. found that polyclonal activated CD4 iTregs, despite
being unable to attenuate GVHD without the addition of rapamycin, also impaired
the capacity of Teffs to clear primary myeloid blast crisis CML. This
impairment was not due to rapamycin administration, as mice treated only with
rapamycin did not succumb to tumor mortality [84]. In our lab, we found
HY-specific iTregs could attenuate GVHD and still maintain the GVL effect, even
against pre-established P815 mastocytoma tumors [87]. However, our recent data
shows that alloreactive CD4 iTregs, when infused three days prior to Teffs,
significantly impairs the GVL effect (unpublished findings). We aim to further
elucidate the mechanism that underlies CD4 iTregs impairment of GVL function.
Improving iTreg
Therapy
CD8 iTregs
A less understood population of suppressor T cells
is derived from the CD8 T-cell lineage [17,18]. Surprisingly, after allogeneic
BMT in murine models, significant populations of CD8+CD25+Foxp3+ iTregs
have been shown to emerge early after transplantation [98,99], but not after
syngeneic transplant. These CD8 iTregs were found to express similar
suppressive molecules as CD4 iTregs (GITR, CD44, CTLA-4, and CD25), and could
potentially be substitutes for CD4 iTregs to attenuate GVHD [98].
Though, CD8 iTregs did express increased levels of
α4β7 when compared to CD4 iTregs [99]. Importantly, when these CD8 iTregs
were isolated from recipient mice and used as a prophylactic in secondary
recipients, they were able to significantly attenuate GVHD [99]. To compare
these findings to human samples, patients’ peripheral blood was analyzed 6
months post-transplant and, surprisingly, no CD8+CD25+Foxp3+ iTregs
were found. Authors later found that all patients had received cyclosporine as
a prophylactic and thus concluded CD8 iTregs were acutely sensitive to
cyclosporine treatment [99]. Future experiments are needed to see if this
population arises in patients receiving various prophylactic therapies, such as
rapamycin.
Currently, only two groups have published
pre-clinical experimental data using in vitro generated CD8
iTregs to attenuate GVHD, with one result contradicting the other. While
testing polyclonal CD4 iTregs, Zhang et al. simultaneously generated polyclonal
CD8 iTregs, and found them to be equally pathogenic due to loss of Foxp3
expression 3 weeks post-transplant [84]. They also found CD8 iTregs to be less
responsive to Foxp3 stabilization, using rapamycin and IL-2 complex treatments,
as compared to CD4 iTregs [84]. Due to the inability to attenuate GVHD, the GVL
function was not assessed in that study. In contrast, CD8 iTreg therapy by
Zheng and colleagues involved isolating naïve human CD8+CD25-CD45RA+CD45RO- and
generating alloreactive CD8 iTregs (termed CD8hi) by stimulating
solely with hCD40-B cells (100).
These CD8hi iTregs potently suppressed GVHD induced by hPBMC
injected into Rag2-/-γc-/- mice (humanized mouse
model of GVHD). Authors used lymphoblastoid cell line (LCL) to assess CD8hi ability
to maintain the GVL effect. Infusion of CD8hi iTregs did not
impair the GVL effect, as LCL tumor was cleared within the blood of recipient
mice versus PBS treated controls [100]. Interestingly, it was found CD8hi iTregs
had direct a cytotoxic effect against LCL tumors through Fas- FasL, perforin,
and granzyme B pathways; inhibition of any of the three negated the lysis of
LCL tumors in vitro. The cytotoxic effect of CD8 iTregs
directly correlates with our own findings using murine alloreactive CD8 iTregs
in GVHD. We found that CD8 iTregs possess some direct toxicity against P815
mastocytoma, yet not enough to fully eradicate tumor without Teff cell infusion
(unpublished findings). Lastly, These CD8 iTregs were moderately effective at
GVHD attenuation.
Two heads are
better than one: Combinational Therapy
The dichotomy we have seen between CD4 and CD8
iTregs, especially with regards to GVL and GVHD responses, raises the question
as to whether these cells can work together to optimize the outcome of
allo-HCT? As our knowledge about cellular and biological processes continues to
expand, clinicians and scientists have moved from a singular approach in order
to incorporate a combinational therapeutic approach in a vast majority of
disease models. Alloreactive CD4 iTregs are able to potently attenuate GVHD,
yet severely compromise GVL function. Alloreactive CD8 iTregs only modestly
attenuate GVHD, but possess GVL capability (unpublished findings). We
hypothesized that combining these two cellular therapies would result in
attenuation of GVHD while preserving the GVL effect. Indeed, we found that in
allogeneic BMTs, a combination of CD4 iTregs and CD8 iTregs was effectively
able to decrease GVHD while maintaining GVL (unpublished findings). The precise
mechanisms underlying the ability of this combination therapy to mediate this
effect are still under investigation. With regards to combinational therapy,
two investigators have found that the addition of Rapamycin and IL-2 complexes
in conjunction with iTreg infusion creates optimal attenuation of GVHD [84,99].
We believe that even more beneficial combinational therapies will emerge for
GVHD in years to come.
Modifying Tregs
Tregs are the
master regulators of balance in our immune systems. Given their natural
function, we have tried to exploit them to control immune disorders
characterized by unbridled inflammation (namely autoimmunity/GVHD). However,
isolation, expansion, and reinfusion of Tregs did not result in an adequate therapy.
Thus, investigators are eagerly testing new strategies to increase the
specificity, stability, and activity of Tregs. Chimeric antigen receptor (CAR)
modified T cells have shown great promise for increasing the antitumor effects
in acute and chronic B cell malignancies [101,102] as well as some solid tumors
[103]. Since Tregs are themselves derived from the same lymphocyte progenitors,
it is tempting to envision the use of CARs to increase Treg specificity and
stability. In this regard, specificity was easily achieved, illustrated by two
studies using hapten-specific CAR Tregs that were more potent in alleviating
experimental colitis than unmodified Tregs [104,105]. Given the increased
potency of antigen-specific iTregs compared to polyclonal iTregs, it would be
ideal to find a way to engineer iTregs that could specifically suppress
responses against tissue damage (GVHD), while ignoring responses to tumor
antigens (GVL). To increase stability, silencing of Stub1, a molecule that
ubquinates Foxp3, was tested by infecting Tregs with lentivirus containing
sh-Stub1 (silencing RNA). It was found that sh-Stub1 Tregs were more stable
during experimental colitis induction [65]. Likewise, Restifo and colleagues
established BACH2 as a key partner for Foxp3 stability, as genetic deletion
resulted in Tregs inability to suppress lethal inflammation in RAG KO mice
[106]. Therefore, Retrovirally induced expression of BACH2 in iTregs could
potentially increase their stability and should be further investigated.
CONCLUDING
REMARKS
The field of
regulatory T cell therapy has come a long way since their discovery in 1970.
However, there is still a long way to go. Although nTregs are an effective
source for therapy, their low proliferative potential remains a major issue.
Additionally, the expansion of GMP-grade nTreg for use in allo-HCT recipients
requires significant clinical infrastructure and coordination. iTregs can be
generated rapidly, but a consensus on their stability and ability to suppress
GVHD has not been reached. With the field trending towards investigating the
differential abilities between CD4 vs. CD8 iTregs with regards to GVHD and GVL
responses, the convergence of these two therapies seems inevitable. The coming
clinical trials involving both alloreactive nTregs and polyclonal iTregs will
give us detailed insight into the next steps for improving iTreg cellular
therapy for the treatment of GVHD. Furthermore, genetically engineering Tregs
opens a new avenue to optimize or tailor Treg therapy in the near future.
ACKNOWLEDGEMENTS
We would like
to thank all past and current members of the Yu Lab for their intellectual
support. The research in the Yu Lab was supported in part by the US National
Institutes of Health (R01 CA11816, CA143812, AI 082685, and CA169116).
1. Pidala J, Lee SJ, Ahn KW, Spellman S, Wang HL, et
al. (2014) Nonpermissive HLA-DPB1 mismatch increases mortality after
myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood
124: 2596-606.
2. Goulmy E, Schipper R, Pool J, Blokland E,
Falkenburg JH, et al. (1996) Mismatches of minor histocompatibility antigens
between HLA-identical donors and recipients and the development of
graft-versus-host disease after bone marrow transplantation. N Engl J Med 334:
281-285.
3. Fu J, Heinrichs J, Yu XZ (2014) Helper T-cell
differentiation in graft-versus-host disease after allogeneic hematopoietic
stem cell transplantation. Arch Immunol Ther Exp (Warsz) 62: 277-301.
4. Pasquini MC ZX (2014) Current uses and outcomes of
hematopoietic stem cell transplantation: 2014 CIBMTR Summary Slides.
5. Brunstein CG, Blazar BR, Miller JS, Cao Q, Hippen
KL, et al. (2013) Adoptive transfer of umbilical cord blood-derived regulatory
T cells and early viral reactivation. Biol Blood Marrow Transplant 19:
1271-1273.
6. Anasetti C (2015) Ex-vivo Expanded Donor Regulatory
T Cells for Prevention of Acute Graft-Versus-Host Disease. clinicaltrails.gov.
7. Veerapathran A, Pidala J, Beato F, Yu XZ, Anasetti
C (2011) Ex vivo expansion of human Tregs specific for alloantigens presented
directly or indirectly. Blood 118: 5671-5680.
8. Gershon RK, Kondo K (1970) Cell interactions in the
induction of tolerance: the role of thymic lymphocytes. Immunology 18: 723-737.
9. Gershon RK, Cohen P, Hencin R, Liebhaber SA (1972)
Suppressor T cells. J Immunol 108: 586-590.
10. Kronenberg M, Steinmetz M, Kobori J, Kraig E, Kapp
JA, et al. (1983) RNA transcripts for I-J polypeptides are apparently not
encoded between the I-A and I-E subregions of the murine major
histocompatibility complex. Proc Natl Acad Sci USA 80: 5704-5708.
11. Sakaguchi S, Takahashi T, Nishizuka Y (1982) Study
on cellular events in post-thymectomy autoimmune oophoritis in mice. II. Requirement
of Lyt-1 cells in normal female mice for the prevention of oophoritis. J Exp
Med 156: 1577-1586.
12. Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M
(1995) Immunologic self-tolerance maintained by activated T cells expressing IL-2
receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance
causes various autoimmune diseases. J Immunol 155: 1151-1164.
13. Sakaguchi S, Miyara M, Costantino CM, Hafler DA
(2010) FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol
10: 490-500.
14. Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3
programs the development and function of CD4+CD25+ regulatory T cells. Nat
Immunol 4: 330-336.
15. Gambineri E, Torgerson TR, Ochs HD (2003)
Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance
(IPEX), a syndrome of systemic autoimmunity caused by mutations of FOXP3, a
critical regulator of T-cell homeostasis. Curr Opin Rheumatol 15: 430-435.
16. Roncarolo MG, Bacchetta R, Bordignon C, Narula S,
Levings MK (2001) Type 1 T regulatory cells. Immunol Rev 182: 68-79.
17. Guillonneau C, Picarda E, Anegon I (2010) CD8+
regulatory T cells in solid organ transplantation. Curr Opin Organ Transplant
15: 751-756.
18. Cortesini R, LeMaoult J, Ciubotariu R, Cortesini NS
(2001) CD8+CD28- T suppressor cells and the induction of antigen-specific,
antigen-presenting cell-mediated suppression of Th reactivity. Immunol Rev 182:
201-206.
19. Talmadge JE, Gabrilovich DI (2013) History of
myeloid-derived suppressor cells. Nat Rev Cancer 13: 739-752.
20. Candando KM, Lykken JM, Tedder TF (2014) B10 cell
regulation of health and disease. Immunol Rev 259: 259-272.
21. Asano M, Toda M, Sakaguchi N, Sakaguchi S (1996)
Autoimmune disease as a consequence of developmental abnormality of a T cell
subpopulation. J Exp Med 184: 387-396.
22. Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck
AE, et al. (2001) Thymic selection of CD4+CD25+ regulatory T cells induced by
an agonist self-peptide. Nat Immunol 2: 301-306.
23. Lio CW, Hsieh CS (2011) Becoming self-aware: the
thymic education of regulatory T cells. Curr Opin Immunol 23: 213-219. Salomon
B, Lenschow DJ, Rhee L, Ashourian N, Singh B, et al. (2000) B7/CD28
costimulation is essential for the homeostasis of the CD4+CD25+
immunoregulatory T cells that control autoimmune diabetes. Immunity 12:
431-440.
24. Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh
B, et al. (2000) B7/CD28 costimulation is essential for the homeostasis of the
CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity
12: 431-440.
25. D'Cruz LM, Klein L (2005) Development and function
of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin
2 signaling. Nat Immunol 6: 1152-1159.
26. Hori S, Nomura T, Sakaguchi S (2003) Control of
regulatory T cell development by the transcription factor Foxp3. Science 299:
1057-1061.
27. Setoguchi R, Hori S, Takahashi T, Sakaguchi S
(2005) Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T
cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2
neutralization. J Exp Med 201: 723-735.
28. Chen W, Jin W, Hardegen N, Lei KJ, Li L, et al.
(2003) Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory
T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med 198:
1875-1886.
29. Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ
(2007) All-trans retinoic acid mediates enhanced T reg cell growth,
differentiation, and gut homing in the face of high levels of co-stimulation. J
Exp Med 204: 1765-1774.
30. Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall
J, Sun CM, et al. (2007) A functionally specialized population of mucosal
CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic
acid-dependent mechanism. J Exp Med 204: 1757-1764.
31. Schmidt A, Oberle N, Krammer PH (2012) Molecular
mechanisms of treg-mediated T cell suppression. Front Immunol 3: 51.
32. Shevach EM, McHugh RS, Piccirillo CA, Thornton AM
(2001) Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol
Rev 182: 58-67.
33. Levine AG, Arvey A, Jin W, Rudensky AY (2014)
Continuous requirement for the TCR in regulatory T cell function. Nat Immunol
15: 1070-1078.
34. Qureshi OS, Zheng Y, Nakamura K, Attridge K,
Manzotti C, et al. (2011) Trans-endocytosis of CD80 and CD86: a molecular basis
for the cell-extrinsic function of CTLA-4. Science 332: 600-603.
35. Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T,
Miyara M, et al. (2008) CTLA-4 control over Foxp3+ regulatory T cell function.
Science 322: 271-275.
36. Liang B, Workman C, Lee J, Chew C, Dale BM, et al.
(2008) Regulatory T cells inhibit dendritic cells by lymphocyte activation
gene-3 engagement of MHC class II. J Immunol 180: 5916-5926.
37. Sarris M, Andersen KG, Randow F, Mayr L, Betz AG
(2008) Neuropilin-1 expression on regulatory T cells enhances their
interactions with dendritic cells during antigen recognition. Immunity 28:
402-413.
38. Solomon BD, Mueller C, Chae WJ, Alabanza LM, Bynoe
MS (2011) Neuropilin-1 attenuates autoreactivity in experimental autoimmune
encephalomyelitis. Proc Natl Acad Sci USA 108: 2040-2045.
39. Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F
(1999) An essential role for interleukin 10 in the function of regulatory T
cells that inhibit intestinal inflammation. J Exp Med 190: 995-1004.
40. Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J,
Castelli L, et al. (2008) Regulatory T cell-derived interleukin-10 limits
inflammation at environmental interfaces. Immunity 28: 546-558.
41. Andersson J, Tran DQ, Pesu M, Davidson TS, Ramsey
H, et al. (2008) CD4+ FoxP3+ regulatory T cells confer infectious tolerance in
a TGF-beta-dependent manner. J Exp Med 205: 1975-1981.
42. Chaturvedi V, Collison LW, Guy CS, Workman CJ,
Vignali DA (2011) Cutting edge: Human regulatory T cells require IL-35 to
mediate suppression and infectious tolerance. J Immunol 186: 6661-6666.
43. Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ
(2007) CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated
apoptosis of effector CD4+ T cells. Nat Immunol 8: 1353-1362.
44. Borsellino G, Kleinewietfeld M, Di Mitri D,
Sternjak A, Diamantini A, et al. (2007) Expression of ectonucleotidase CD39 by
Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression.
Blood 110: 1225-1232.
45. Kobie JJ, Shah PR, Yang L, Rebhahn JA, Fowell DJ,
et al. (2006) T regulatory and primed uncommitted CD4 T cells express CD73,
which suppresses effector CD4 T cells by converting 5'-adenosine monophosphate
to adenosine. J Immunol 177: 6780-6786.
46. Ernst PB, Garrison JC, Thompson LF (2010) Much ado
about adenosine: adenosine synthesis and function in regulatory T cell biology.
J Immunol 185: 1993-1998.
47. Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle
RJ (2005) Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory
cells involves a granzyme B-dependent, perforin-independent mechanism. J
Immunol 174: 1783-1786.
48. Cao X, Cai SF, Fehniger TA, Song J, Collins LI, et
al. (2007) Granzyme B and perforin are important for regulatory T cell-mediated
suppression of tumor clearance. Immunity 27: 635-646.
49. Cai SF, Cao X, Hassan A, Fehniger TA, Ley TJ (2010)
Granzyme B is not required for regulatory T cell-mediated suppression of
graft-versus-host disease. Blood 115: 1669-1677.
50. Velaga S, Ukena SN, Dringenberg U, Alter C, Pardo
J, et al. (2015) Granzyme A Is Required for Regulatory T-Cell Mediated
Prevention of Gastrointestinal Graft-versus-Host Disease. PLoS One 10:
e0124927.
51. Hoffmann P, Ermann J, Edinger M, Fathman CG,
Strober S (2002) Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal
acute graft-versus-host disease after allogeneic bone marrow transplantation. J
Exp Med 196: 389-399.
52. Wysocki CA, Jiang Q, Panoskaltsis-Mortari A, Taylor
PA, McKinnon KP, et al. (2005) Critical role for CCR5 in the function of donor
CD4+CD25+ regulatory T cells during acute graft-versus-host disease. Blood 106:
3300-3307.
53. Taylor PA, Panoskaltsis-Mortari A, Swedin JM, Lucas
PJ, Gress RE, et al. (20040 L-Selectin(hi) but not the L-selectin(lo) CD4+25+
T-regulatory cells are potent inhibitors of GVHD and BM graft rejection. Blood
104: 3804-3812.
54. Ermann J, Hoffmann P, Edinger M, Dutt S,
Blankenberg FG, et al. (2005) Only the CD62L+ subpopulation of CD4+CD25+
regulatory T cells protects from lethal acute GVHD. Blood 105: 2220-2226.
55. Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C,
Martinez-Llordella M, et al. (2009) Instability of the transcription factor
Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol
10: 1000-1007.
56. Rubtsov YP, Niec RE, Josefowicz S, Li L, Darce J,
et al. (2010) Stability of the regulatory T cell lineage in vivo. Science 329:
1667-1671.
57. Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, et
al. (2009) Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T
cells upon repetitive in vitro stimulation. Eur J Immunol 39: 1088-1097.
58. Duarte JH, Zelenay S, Bergman ML, Martins AC,
Demengeot J (2009) Natural Treg cells spontaneously differentiate into
pathogenic helper cells in lymphopenic conditions. Eur J Immunol 39: 948-955.
59. Sawant DV, Vignali DA (2014) Once a Treg, always a
Treg? Immunol Rev 259: 173-191
60. Hori S. 2014. Lineage stability and phenotypic plasticity
of Foxp3(+) regulatory T cells. Immunol Rev 259: 159-172.
61. Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush
K, et al. (2010) Role of conserved non-coding DNA elements in the Foxp3 gene in
regulatory T-cell fate. Nature 463: 808-812.
62. Ohkura N, Hamaguchi M, Morikawa H, Sugimura K,
Tanaka A, et al. (2012) T cell receptor stimulation-induced epigenetic changes
and Foxp3 expression are independent and complementary events required for Treg
cell development. Immunity 37: 785-799.
63. Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, et al.
(2012) A multiply redundant genetic switch 'locks in' the transcriptional
signature of regulatory T cells. Nat Immunol 13: 972-980.
64. Morawski PA, Mehra P, Chen C, Bhatti T, Wells AD
(2013) Foxp3 protein stability is regulated by cyclin-dependent kinase 2. J
Biol Chem 288: 24494-24502.
65. Chen Z, Barbi J, Bu S, Yang HY, Li Z, et al. (2013)
The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive
activity by promoting degradation of the transcription factor Foxp3. Immunity
39: 272-285.
66. Huynh A, DuPage M, Priyadharshini B, Sage PT,
Quiros J, et al. (2015) Control of PI(3) kinase in Treg cells maintains
homeostasis and lineage stability. Nat Immunol 16: 188-196.
67. Shrestha S, Yang K, Guy C, Vogel P, Neale G, et al.
(2015) Treg cells require the phosphatase PTEN to restrain TH1 and TFH cell
responses. Nat Immunol 16: 178-187.
68. DuPage M, Chopra G, Quiros J, Rosenthal WL, Morar
MM, et al. (2015) The chromatin-modifying enzyme Ezh2 is critical for the
maintenance of regulatory T cell identity after activation. Immunity 42:
227-238.
69. Yang XP, Jiang K, Hirahara K, Vahedi G, Afzali B,
et al. (2015) EZH2 is crucial for both differentiation of regulatory T cells
and T effector cell expansion. Sci Rep 5: 10643.
70. He S, Xie F, Liu Y, Tong Q, Mochizuki K, et al.
(2013) The histone methyltransferase Ezh2 is a crucial epigenetic regulator of
allogeneic T-cell responses mediating graft-versus-host disease. Blood 122:
4119-4128.
71. Taylor PA, Lees CJ, Blazar BR (2002) The infusion
of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells
inhibits graft-versus-host disease lethality. Blood 99: 3493-3499.
72. Edinger M, Hoffmann P, Ermann J, Drago K, Fathman
CG, et al. (2003) CD4+CD25+ regulatory T cells preserve graft-versus-tumor
activity while inhibiting graft-versus-host disease after bone marrow
transplantation. Nat Med 9: 1144-1150.
73. Levings MK, Sangregorio R, Roncarolo MG (2001)
Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell
proliferation and can be expanded in vitro without loss of function. J Exp Med
193: 1295-1302.
74. Godfrey WR, Ge YG, Spoden DJ, Levine BL, June CH,
et al. (2004) In vitro-expanded human CD4(+)CD25(+) T-regulatory cells can
markedly inhibit allogeneic dendritic cell-stimulated MLR cultures. Blood 104:
453-461.
75. Godfrey WR, Spoden DJ, Ge YG, Baker SR, Liu B, et
al. (2005) Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express
FoxP3 protein and manifest potent suppressor function. Blood 105: 750-758.
76. Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad
D, et al. (2011) Massive ex vivo expansion of human natural regulatory T cells
(T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 3:
83ra41.
77. Veerapathran A, Pidala J, Beato F, Betts B, Kim J,
et al. (2013) Human regulatory T cells against minor histocompatibility
antigens: ex vivo expansion for prevention of graft-versus-host disease. Blood
122: 2251-2261.
78. Trzonkowski P, Bieniaszewska M, Juscinska J,
Dobyszuk A, Krzystyniak A, et al. (2009) First-in-man clinical results of the
treatment of patients with graft versus host disease with human ex vivo
expanded CD4+CD25+CD127- T regulatory cells. Clin Immunol 133: 22-26.
79. Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen
KL, et al. (2011) Infusion of ex vivo expanded T regulatory cells in adults
transplanted with umbilical cord blood: safety profile and detection kinetics.
Blood 117: 1061-1070.
80. Di Ianni M, Falzetti F, Carotti A, Terenzi A,
Castellino F, et al. (2011) Tregs prevent GVHD and promote immune
reconstitution in HLA-haploidentical transplantation. Blood 117: 3921-3928.
81. Davidson TS, DiPaolo RJ, Andersson J, Shevach EM
(2007) Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of
Foxp3+ T regulatory cells. J Immunol 178: 4022-4026.
82. Fantini MC, Becker C, Monteleone G, Pallone F, Galle
PR, et al. (2004) Cutting edge: TGF-beta induces a regulatory phenotype in
CD4+CD25- T cells through Foxp3 induction and down-regulation of Smad7. J
Immunol 172: 5149-5153.
83. Beres A, Komorowski R, Mihara M, Drobyski WR (2011)
Instability of Foxp3 expression limits the ability of induced regulatory T
cells to mitigate graft versus host disease. Clin Cancer Res 17: 3969-3983.
84. Zhang P, Tey SK, Koyama M, Kuns RD, Olver SD, et
al. (2013) Induced regulatory T cells promote tolerance when stabilized by
rapamycin and IL-2 in vivo. J Immunol 191: 5291-5303.
85. Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis
NC, et al. (2011) Generation and large-scale expansion of human inducible
regulatory T cells that suppress graft-versus-host disease. Am J Transplant 11:
1148-1157.
86. Semple K, Yu Y, Wang D, Anasetti C, Yu XZ (2011)
Efficient and selective prevention of GVHD by antigen-specific induced Tregs
via linked-suppression in mice. Biol Blood Marrow Transplant 17: 309-318.
87. Li J, Heinrichs J, Haarberg K, Semple K,
Veerapathran A, et al. (2015) HY-Specific Induced Regulatory T Cells Display
High Specificity and Efficacy in the Prevention of Acute Graft-Versus-Host
Disease. J Immunol.
88. Koenecke C, Czeloth N, Bubke A, Schmitz S,
Kissenpfennig A, et al. (2009) Alloantigen-specific de novo-induced Foxp3+ Treg
revert in vivo and do not protect from experimental GVHD. Eur J Immunol 39:
3091-3096.
89. Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM
(2011) Dendritic cells induce antigen-specific regulatory T cells that prevent
graft versus host disease and persist in mice. J Exp Med 208: 2489-2496.
90. Zhao C, Shi G, Vistica BP, Hinshaw SJ, Wandu WS, et
al. (2014) Induced regulatory T-cells (iTregs) generated by activation with
anti-CD3/CD28 antibodies differ from those generated by the physiological-like
activation with antigen/APC. Cell Immunol 290: 179-184.
91. Gao W, Lu Y, El Essawy B, Oukka M, Kuchroo VK, et
al. (2007) Contrasting effects of cyclosporine and rapamycin in de novo
generation of alloantigen-specific regulatory T cells. Am J Transplant 7:
1722-1732.
92. Lu L, Ma J, Li Z, Lan Q, Chen M, et al. (2011)
All-trans retinoic acid promotes TGF-beta-induced Tregs via histone
modification but not DNA demethylation on Foxp3 gene locus. PLoS One 6: e24590.
93. Nguyen VH, Zeiser R, Dasilva DL, Chang DS, Beilhack
A, et al. (2007) In vivo dynamics of regulatory T-cell trafficking and survival
predict effective strategies to control graft-versus-host disease following
allogeneic transplantation. Blood 109: 2649-2656.
94. MacMillan M (2015) Inducible Regulatory T Cells
(iTregs) in Non-Myeloablative Sibling Donor Peripheral Blood Stem Cell
Transplantation.
95. Nishikawa H, Jager E, Ritter G, Old LJ, Gnjatic S
(2005) CD4+ CD25+ regulatory T cells control the induction of antigen-specific
CD4+ helper T cell responses in cancer patients. Blood 106: 1008-1011.
96. Shimizu J, Yamazaki S, Sakaguchi S (1999) Induction
of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor
immunity and autoimmunity. J Immunol 163: 5211-5218.
97. Trenado A, Charlotte F, Fisson S, Yagello M,
Klatzmann D, et al. (2003) Recipient-type specific CD4+CD25+ regulatory T cells
favor immune reconstitution and control graft-versus-host disease while
maintaining graft-versus-leukemia. J Clin Invest 112: 1688-1696.
98. Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ,
Williams CB, et al. (2012) CD8+ Foxp3+ regulatory T cells are induced during
graft-versus-host disease and mitigate disease severity. J Immunol 189:
464-474.
99. Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt
NC, et al. (2012) Identification and expansion of highly suppressive
CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow
transplantation. Blood 119: 5898-5908.
100. Zheng J, Liu Y, Liu M, Xiang Z, Lam KT, et al. (2013) Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Sci Transl Med 5: 168ra9.
101. Porter DL, Kalos M, Zheng Z, Levine B, June C (2011) Chimeric Antigen Receptor Therapy for B-cell Malignancies. J Cancer 2: 331-332.
102. Kalos M, Levine BL, Porter DL, Katz S, Grupp SA (2011) T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 3: 95ra73.
103. Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, et al. (2008) Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 14: 1264-1270.
104. Elinav E, Adam N, Waks T, Eshhar Z (2009) Amelioration of colitis by genetically engineered murine regulatory T cells redirected by antigen-specific chimeric receptor. Gastroenterology 136: 1721-1731.
105. Elinav E, Waks T, Eshhar Z (2008) Redirection of regulatory T cells with predetermined specificity for the treatment of experimental colitis in mice. Gastroenterology 134: 2014-2024.
106. Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, et al. (2013) BACH2 represses effector programs to stabilize T(reg)-mediated immune homeostasis. Nature 498: 506-510.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Alcoholism Clinical Research
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Spine Diseases
- International Journal of Surgery and Invasive Procedures (ISSN:2640-0820)
- Journal of Clinical Trials and Research (ISSN:2637-7373)
- Journal of Forensic Research and Criminal Investigation (ISSN: 2640-0846)