772
Views & Citations10
Likes & Shares
Background:
Tuberculosis (TB) is a highly infectious disease that classified as a major
global health problem and to protect against the infection two French
scientists develop a live attenuated vaccine that called Bacillus of
Calmette-Guerin. Before vaccination, sometime Tuberculin test is to indicate
previous exposure to TB.
Objective: This
study was aimed to determine the efficacy of BCG vaccine by a screening of
healthy, vaccinated adult’s subjects in Khartoum capital of Sudan.
Method: A
total of one hundred (n=100) healthy participant were introduced in this study.
The participants were involving 55 (55%) males and 45 (45%) female. In
addition, they were over 20 years and most of them had a scar in their
vaccination site. Only those whom TB symptoms free participants were included
and screened by manteaux test. The manteaux test was done through injection of
each participant by purified protein derivative PPD (only 0.1ml) intradermally
into his volar forearm, then 48-72 post-injection the induration was observed
and the diameter was measured.
Results: The
results showed that out of one hundred (n=100) participants screened, only 39
(39%) were positive for Manteaux test (≥ 10 mm diameter), while 61 (61%) were
negative (≤ 10 mm diameter). Among the 39 positives, 33 show reading between
10mm to 15mm and 6 of them show zone ≥ 15 mm. Among 61 tuberculin test,
negative participants 53 were showed no induration post PPD injection and the
rest were shows reading zone between 5 to 9 mm. Furthermore, the result shows
that among the 39 positive participants 23 (58.97%) were male while only 16
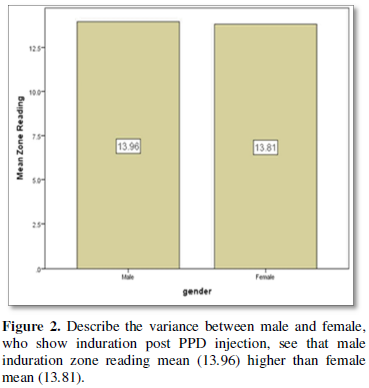
(41.03%) were female. The mean of zone reading among the positive participants
is higher in male 13.96 ± 3.29 than female 13.81 ± 2.22.
Conclusion: The
study concluded that more than half of the participants were negative for
tuberculin test and this may be interpreted by either the vaccine was invalid
at the time of vaccination or their cell-mediated immunity against TB is
reduced. Moreover, the discrepancy in the means of the zone reading between
male and female may be related to some physiological difference. Further
studies with more sample size and by using a more advanced technique (IFNγ
measurement) should be done to clarify the results.
Keywords: Tuberculosis;
BCG; Manteaux test; Cell mediated immunity
INTRODUCTION
Diagnosis of active TB is based on chest X-rays, as well as microscopic
examination and culture of body fluids besides polymerase chain reaction, while
the diagnosis of latent TB based on the tuberculin skin test (TST) or blood
tests [12]. In patients with drug-susceptible TB, a 6 months rifampicin-based
regimen (2 months of isoniazid, rifampicin, pyrazinamide and ethambutol,
followed by 4 months of isoniazid and rifampicin) should be used. MDR-TB
(multidrug-resistant tuberculosis) is caused by bacteria that do not respond to
the 2 most powerful first-line anti-TB drugs, isoniazid, and rifampicin. MDR-TB
and rifampicin-resistant TB (RR-TB) are treatable using second-line treatment
options which are limited with respect to availability and efficacy and require
treatment of considerably longer duration [13].
TB is caused by the infectious agent known as Mycobacterium
tuberculosis (MTB), this rod-shaped bacterium also called Koch’s bacillus, was
discovered by Dr. Robert Koch in 1882 [14]. MTB is a unique acid-fast
bacterium. It is unique because it is high lipid and mycolic acid content of
its cell wall. The physiology of M. tuberculosis is highly aerobic and requires
high levels of oxygen [15].
TB is the ninth leading cause of death worldwide and the leading cause
from a single infectious agent, ranking above HIV/AIDS. In 2016, there were an
estimated 1.3 million TB deaths among HIV-negative people (down from 1.7
million in 2000) and an additional 374 000 deaths among HIV-positive people
[15]. In 2016, 2.5 million people fell ill with TB in the African region,
accounting for a quarter of new TB cases worldwide. An estimated 417,000 people
died from the disease in the African region (1.7 million globally) in 2016.
Over 25% of TB deaths occur in the African Region [16].
In Sudan, the tuberculosis-related mortality rate is estimated at 25.0
per 100 000 population. A total of 20 181 detected tuberculosis cases were
reported in 2013, of which 5980 (30%) were new sputum smear-positive cases. The
treatment success rate of new and relapsed cases registered in 2012 was 75.0%.
Drug-resistant tuberculosis is estimated at 1.9% among new cases and 20.0%
among previously treated cases [17].
TB is the most unpardonable infectious disease and the most common one,
which easily spread. Bacillus of Calmette-Guerin (BCG) is the only successful
TB vaccine [18]. The BCG vaccine was developed over the period of 13 years from
(1908-1921) its live vaccine derived from the strain of Mycobacterium bovis. That was attenuated by Calmette and Guerin at
Pasteur Institute in Lille France. And it was first administrated to a human in
1921 [13]. The BCG is usually given intramuscular to babies and children birth
up to the age of 16; it’s also sometimes given to adult up to the age of 35
years. But the vaccine does not work well in adults; the adults are often given
skin test before vaccine [13]. The rate of protective efficacy of BCG vaccine
has been affected by the method, route of administration environment and
characteristic of the population [13].
he standard dose of BCG vaccine is 0.05 mL of the reconstituted vaccine
for infants aged 1 year. BCG vaccines must be administered by intradermal
injection. Correct intradermal administration can be verified by bleb
formation. BCG vaccine should be injected in a clean healthy area of skin. The
vaccine should be given preference in the lateral aspect of the upper arm.
There are no published data on efficacy/effectiveness and safety for other
anatomic sites of administration. Among the many available BCG vaccine
products, there is no preferred product for use, in any age or risk group [13].
About 95% of BCG vaccine recipients experience a reaction at the
injection site characterized by a papule which may progress to become
ulcerated, with healing after 2-5 months leaving a superficial scar. This is
considered normal. Adverse events following immunization (AEFI) with BCG are
dependent on a number of factors including the strain used in the vaccine, the
number of viable bacilli in the batch, and variation in injection technique.
Severe AEFI includes local reactions such as injection site abscess, severe
ulceration or suppurative lymphadenitis usually caused by inadvertent injection
of the vaccine sub-dermally. The advent of molecular tests has facilitated the
identification of rare events, such as disseminated BCG disease that may occur
between 1.56 and 4.29 cases per million doses [13].
The efficacy of BCG remains to vary from 0%-80% [21]. Its 70%-80%
effective against the most severe form of T.B such as T.B meningitis. It’s less
effective in preventing the form of T.B that affect the lung but it’s still considered
important strategies in countries with high burden of tuberculosis because it’s
benefit to the infant but it’s affecting the control of T.B has been limited
[22].
The immune response to mycobacterial infection is predominantly
cellular [23]. It is highly dependent upon gamma interferon (IFN-γ) production
by macrophages and antigen-specific T cells [24].
The Manteaux Test (MT) is a classical delayed-type hypersensitivity
(DTH) response to the intradermal injection of tuberculin purified protein derivative
(PPD). It represents a cutaneous T cell-mediated memory recall immune response.
The manteaux test is also known as Tuberculin skin test has been the
traditional method for detection of infection with tubercle bacilli (latent
infection) [23] it was performed by using 5 TU (tuberculin unit) equivalent to
0.1 ml of tuberculin PPD RT23 [24]. The manteaux test assesses the patient's
response to a stimulus of purified protein derivative (PPD) 0.1 mL is injected
intradermally into the volar forearm to produce a wheel of 6-10 mm diameter
[24]. After 48-72 h the induration is measured in millimetres at the point of
injection and interpreted according to current guidelines [25]. To get a
reliable reading of the manteaux skin test usually standardization of procedures,
training, supervision, and practice are required [25]. The results of manteaux
test must be interpreted carefully. The person's medical risk factors determine
the size of induration the result is positive (5 mm, 10 mm or 15 mm) [25].
Monteux test is a sensitive but non-specific in the diagnosis of active
tuberculosis. The interpretation of Monteux needs to be correlated to the
patient’s clinical context [25].
Mantoux test has been also used for a long time as vaccination marker
when there is no previous household contact with tuberculosis or history of
infection so the positive reaction may be a useful signal of cell-mediated
immunity against TB. This study was sought to describe the immune response to
BCG vaccine among healthy, vaccinated adults.
MATERIALS AND
METHODS
This study was a cross-sectional hospital-based conducted in Khartoum
state in ALSHAB HOSPITAL, during the period of January to July 2018. A total of
one hundred participants (n=100) were incorporated in this study. All
participants were adult, healthy, vaccinated most of them had a scar in their
vaccine injecting site. The participants were free of tuberculosis, HIV, renal
disease, other mycobacterial infection also they are not Injectable drug users
or mycobacteriology lab personnel and have no history of tuberculosis disease
or TB household contact, so that the presence of zone may indicate the immunity
against TB. All participants were screened by using the manteaux test.
The procedure of
manteaux test
Monteux testing was performed using 5 TU (tuberculin unit) of
tuberculin PPD RT23 through injection into the forearm. Results were read
within 48 and 72 h post injection and recorded as the transverse diameter (in
mm) of palpable induration. History of BCG vaccination has been taken.
Interpretation of
results
Once the manteaux test used for the diagnosis of latent tuberculosis
the result should be interpreted carefully. In stat of no previous exposure to
the TB infection and no immune system dysfunction, the vaccinated adult should
be developed delayed-type hypersensitivity reaction resulting in induration
zone reading more than 10 mm.
Quality control and
of the results
PPD reagent which used in this test was checked for storage, stability
and reconstituted before starting work.
The method used for
data collection
Data was collected by using administrated questionnaire include the
gender and age.
DATA ANALYSIS
The data that collected from questionnaire and laboratory results were
analysed by SPSS version 15 computerized programs.
RESULTS
DISCUSSION
Currently,
Bacillus Calmette-Guerin (BCG) is the only vaccine approved by FDA for use to
prevent TB. Immunologically, following the BCG intradermal inoculation,
resident epidermal macrophages interact with BCG via several
pattern-recognition receptors (PRRs) resulting in Stimulation of T lymphocytes
and protective immunity [26,27]. The tuberculin skin test (TST) is used as a
diagnostic tool to assess the latent infection with Mycobacterium tuberculosis.
But, it was also widely used as BCG vaccination indicator. The interpretation
of TST result for vaccinated adults remains controversial because the exposure
to the TB antigen may give a false positive reaction for unvaccinated
individuals.
The present study
was aimed to determine the immune response to BCG vaccine among healthy,
vaccinated adults in Khartoum state by using mantoux test and to avoid false
positive and false negative result all participants were selected carefully,
they were free of tuberculosis, HIV, renal disease, other mycobacterial
infection also they are not Injectable drug users or Mycobacteriology lab
personnel and have no history of tuberculosis disease or household TB contact,
so that the presence of zone and induration may be used as good indicator of
the immunity against TB.
The results showed
that out one hundred (n=100) participants screened, only 39 (39%) were positive
for Manteaux test (show ≥ 10 mm induration diameter), while 61 (61%) were
negative (≤ 10 mm induration diameter). These results reflect relatively
intermediate BCG efficacy rate but we must be taken in consideration the fact
that the absence of induration zone among vaccinated adults after manteaux test
is not clear-cut for loss of cell-mediated immunity against TB. Besides, the
manteaux test is only screening approaches and gives the only Idea about the
immunity status of the vaccinated person. So, we need to use standard IFNγ
measurement to clarify the result because CD4+ T cells, as well as the
cytokines IL-12, IFN-γ and TNF, are critical in the control of Mycobacterium
tuberculosis. Furthermore, interferon-gamma release assays (IGRAs) have become common
in clinical use in the 2010s and in some contexts they are used instead of
TSTs, whereas in other contexts TSTs and IGRAs both continue to be useful.
It is worth
mentioning that most participants have a scare in their vaccine injecting site
and this is a very strong evidence that the person has been mount immunity
against TB but antithesis to that our result demonstrated more than half of the
participants were negative to manteaux test it is not surprising when we take
the fact that the immunity to TB has waned with increasing age in
consideration. In our study group only do this work to give a picture about the
efficacy of BCG vaccine among healthy vaccinated participants in Khartoum state
and in order to clarify the picture we need to use an advanced technique like
interferon gamma measurement.
CONCLUSION
We concluded that
the efficacy rate of BCG vaccine is intermediate, male were more respond well
to the vaccine than female, in addition, more than half of participants failed
to develop any induration or zones. Further, studies with more sample size and
using more advanced techniques (IFNγ measurement) should be done to clarify the
results.
ACKNOWLEDGEMENT
1. WHO
(2015) Global Tuberculosis Report 2015. 20th edn.
2. WHO
(2015) Tuberculosis Factsheet.
3. Mandell
G, Bennett J, Dolin R (2010) Mandell, Douglas and Bennett's principles and
practice of infectious diseases. 7th edn. Philadelphia, PA:
Churchill Livingstone, Elsevier, p: 250.
4. CDC
(2012) Basic TB facts. Retrieved February 06, 2016.
5. Center
for Disease Control (2018) How TB spreads? Retrieved March 14, 2018.
6. Kumar
V, Abbas AK, Fausto N, Mitchell RN (2007). Robbins Basic Pathology. 8th
edn. Saunders Elsevier, pp: 516-522.
7. Skolnik
R (2011). Global health 101. 2nd edn. Burlington, MA: Jones &
Bartlett Learning, p: 253.
8. Mainous
AG III, Pomeroy C (2009) Management of antimicrobials in infectious diseases:
Impact of antibiotic resistance. (2nd rev. ed.) Totowa, N.J.: Humana
Press, p: 74.
9. Khan
(2011) Essence of Paediatrics. Elsevier, India, p: 401.
10. Agarwal
R, Malhotra P, Awasthi A, Kakkar N, Gupta D (2005) Tuberculosis dilated
cardiomyopathy: An under-recognized entity? BMC Infect Dis 5: 29.
11. Bozzano
F, Marras F, De Maria A (2014) Immunology of tuberculosis. Mediterr J Hematol
Infect Dis 6: e2014027.
12. Konstantinos
A (2010) Testing for tuberculosis. Australian Prescriber 33: 12-18.
13. World
Health Organisation (2018) BCG vaccines: WHO position paper, February 2018 -
Recommendations. Vaccine 36: 3408-3410.
14. Ryan
KJ, Ray CG (2014) Sherris medical microbiology. 4th edn. MC Graw Hill.
15. Meena
R, Meena LS (2011) Unique characteristics feature of MTB in relation to the immune
system. Am J Immunol 7: 1-8.
16. WHO
(2017) Global tuberculosis report 2017. Accessed from:
www.who.int/tb/publications/global_report/
17. http://www.emro.who.int/sdn/programmes/stop-TB-sudan.html
18. Vienna
(2007) Accelerating advocacy on TB/HIV.
19. Nguipdop-Djomo
P, Heldal E, Rodrigues LC, Abubakar I, Mangtani P (2016) Duration of BCG
protection against tuberculosis and change in effectiveness with time since
vaccination in Norway: A retrospective population-based cohort study. Lancet
Infect Dis 16: 219-226.
20. Aronson
NE, Santosham M, Comstock GW, Howard RS, Moulton LH, et al. (2004) Long-term
efficacy of BCG vaccine in American Indians and Alaska natives: A 60 year
follow-up study. JAMA 291: 2086-2091.
21. Peter
A, Timothy M (2005) The success and failure of BCG - Implications for a novel
TB vaccine. Microbiology 3: 656-662.
22. Ahmad
NA, Abd Hamid HA, Sahril N, Mohd Yusoff MF, Naidu BM, et al. (2013) Bacille
Calmette-Guerin (BCG) revaccination: Is it beneficial for tuberculosis control?
2: 656.
23. Chadha
VK (2001) Tuberculin test. Indian J Pediatr 68: 53-58.
24. Kiwanuka
JP (2005) Interpretation of tuberculin skin-test results in the diagnosis of
tuberculosis in children. Afr Health Sci 5: 152-156.
25. Surajit
N, Basanti A (2012) Mantoux test and its interpretation. Indian Dermatol 3:
2-6.
26. Juan
IM, Joanne T, Jordi BT (2017) Immune responses to Bacillus Calmette-Guérin
vaccination: Why do they fail to protect against Mycobacterium tuberculosis?
Front Immunol 8: 407.
27. Sendide
K, Reiner NE, Lee JS, Bourgoin S, Talal A, et al. (2005) Cross-talk between
CD14 and complement receptor 3 promotes phagocytosis of mycobacteria:
Regulation by phosphatidylinositol 3-kinase and cytohesin-1. J Immunol 174:
4210-4219.
28. Moliva
JI, Turner J, Torrelles JB (2015) Prospects in Mycobacterium bovis Bacillus
Calmette-Guerin (BCG) vaccine diversity and delivery: Why does BCG fail to
protect against tuberculosis? Vaccine 33: 5035-5041.
29. Shen
Y, Zhou D, Qiu L, Lai X, Simon M, et al. (2002) Adaptive immune response of
Vgamma2Vdelta2+ T cells during mycobacterial infections. Science 295:
2255-2258.
30. Afonso-Barroso
A, Clark SO, Williams A, Rosa GT, Nobrega C, et al. (2013) Lipoarabinomannan
mannose caps do not affect mycobacterial virulence or the induction of
protective immunity in experimental animal models of infection and have minimal
impact on in vitro inflammatory
responses. Cell Microbiol 15: 660-674.
31. Sidobre
S, Nigou J, Puzo G, Riviere M (2000) Lipoglycans are putative ligands for the
human pulmonary surfactant protein A attachment to mycobacteria. J Biol Chem
275: 2415-2422.
32. Rosenthal
SR, Loewinsohn E, Graham ML, Liveright D, Thorne G, et al. (1961) BCG
vaccination against tuberculosis in Chicago: A twenty year study statistically
analyzed. Pediatrics 28: 622-641.
33. Smith
PG (1987) Case-control studies of the efficacy of BCG against tuberculosis. In:
International Union against Tuberculosis, ed. Proceedings of the XXVIth IUAT
World Conference on Tuberculosis and Respiratory Diseases. Singapore:
Professional Postgraduate. Services International, pp: 73-79.
34. Clemens
JD, Chuong JJ, Feinstein AR (1983) The BCG controversy: A methodological and
statistical reappraisal. JAMA 249: 2362-2369.