Research Article
How Does Changing Reagent Instrument Combination Affect INR?
1201
Views & Citations201
Likes & Shares
Background: International normalized ratio (INR) was designed to standardize prothrombin time variation for a better monitoring of patients on anti-vitamin K medication. However, several studies have shown that INR might vary with different reagent/instrument combinations.
Aims: To assess the agreement between INR values obtained with three different thromboplastin/instrument combinations.
Methods: INR was measured on plasmas from 331 patients undergoing anti-vitamin K treatment using two reagent/instrument combinations: Both Neoptimal and Neoplastine CI plus on STA-R instrument from Diagnostica STAGO, Asnières, France and Innovinon SYSMEX 2100i instrument from Siemens Health Care Diagnostics, Marbung, Germany. The agreement for each reagent/instrument combination was evaluated using the Bland-Altman plot and Cohen’s Kappa coefficient.
Results: When comparing INR values obtained with both Neoptimal and Neoplastine CI plus, the mean difference was -0.01 and the agreement limits were (-1.16 to 0.96). Cohen’s kappa coefficient was 0.77. For INR values superior to 4.5, the mean difference was 0.26 (-1.25 to 1.77). For the second combination (Neoptimal vs Innovin), the mean difference was -0.28 (-2.16 to 1.6). Cohen’s kappa coefficient was 0.76. When INR was superior to 4.5, the mean difference was -0.7 (-4.3 to 2.9).
Conclusion: The agreement limits between Neoptimal and Neoplastine CI plus are small enough to consider that the reagent can be used interchangeably which is supported by a substantial agreement as shown by Cohen’s kappa coefficient. This is not the case for the couple Neoptimal and innovin in which the agreement limits are wider, particularly when INR values are superior to 4.5. An adjustment in anticoagulant dosage might be necessary.
Keywords: Thromboplastin, Prothrombin time, Standardization, Antivitamin K, Monitoring
Abbreviations: INR: International Normalized Ratio; ISI: International Sensitivity Index; PT: Prothrombin Time
WHAT IS KNOWN AND OBJECTIVE
It is admitted that prothrombin time is affected by thromboplastin reagent. This would be an issue for patients undergoing antivitamin K medication which needs a reliable test for its monitoring. International normalized ratio (INR) was designed in 1983 to standardize prothrombin time variations. The INR is the prothrombin time determined using a reagent with an international sensitivity index (ISI) value of one. The ISI is provided by the manufacturer and is determined by comparing their agent to international reference thromboplastin. However, several studies have shown that the INR might vary depending on the reagent/instrument combination despite using specific ISI [1-3]. Our previous study has compared side by side different combinations and has found that it is not possible to obtain the same INR although the agreement was high enough to be considered clinically acceptable [4]. Currently, new thromboplastins have appeared, and it is important to compare them to existing reagents used routinely. The aim of this study was to evaluate the agreement between INR values obtained with "NEOPTIMAL", a new thromboplastin and two other existing reagents.
MATERIALS & METHODS
Blood samples
Blood samples were collected from 331 patients undergoing long-term antivitamin K therapy (ACENOCOUMAROL, SINTROM® MEDIUS AG, SUISSE) over 6 days. Blood samples were drawn through venipuncture into plastic tubes containing 3.2% sodium citrate with blood/anticoagulant ratio of9/1. Plasma was obtained after centrifugation of blood samples at 2500 g/min for 10 minutes at room temperature. Assays were performed within the following 6 hr.
Reagent and instruments
Three commercial thromboplastin reagents were assayed using two instruments: -Neoptimal and Neoplastine CI plus on STA-R instrument from DIAGNOSTICA STAGO, ASNIÈRES, FRANCE -Innovin on SYSMEX 2100i instrument from SIEMENS HEALTH CARE DIAGNOSTICS, MARBUNG, GERMANY (Table 1).
Clotting assays
For Innovin/Sysmex 2100i combination, INR was calibrated using six lyophilized calibrant plasmas with assigned INR values (PT MULTI CALIBRATORS, SIEMENS HEALTH CARE DIAGNOSTICS, MARBUNG, GERMANY). For Neoptimal and Neoplasitine CI plus on STA-R, PT was precalibrated and INR was calculated using the manufacturer’s ISI (Table 1).
INR values obtained with the different combinations were grouped into four classes used in clinical practice: inferior to 2, [2-3], (3-4.5) and superior to 4.5.

Statistical analysis
Statistical analysis was performed using MedCalc statistical software (version 17.4). The agreement between the INR values obtained through the different thromboplastin/instrument combinations was analyzed using two statistical measurements. (i) The Bland-Altman plot was used when considering INR values as quantitative variables [5]. This method investigates the difference between two measurements. For each blood sample, the mean of INR values obtained with two different instrument/reagent combinations was represented as the abscissa whereas their difference was represented as the ordinate. The mean difference is the estimated bias between the two assays. The “limits of agreement” refers to the mean difference ±2standard deviations. If the differences within the limits of agreement are not clinically important, we could use the two measurement methods interchangeably. (ii) Cohen’s kappa coefficient was used when considering the INR values as qualitative variables [6]. Cohen’s kappa coefficient measures the agreement between qualitative variables. INR values were grouped into different classes. A higher agreement is represented by a value closer to one.
RESULTS
Neoptimal vs Neoplastine CI plus
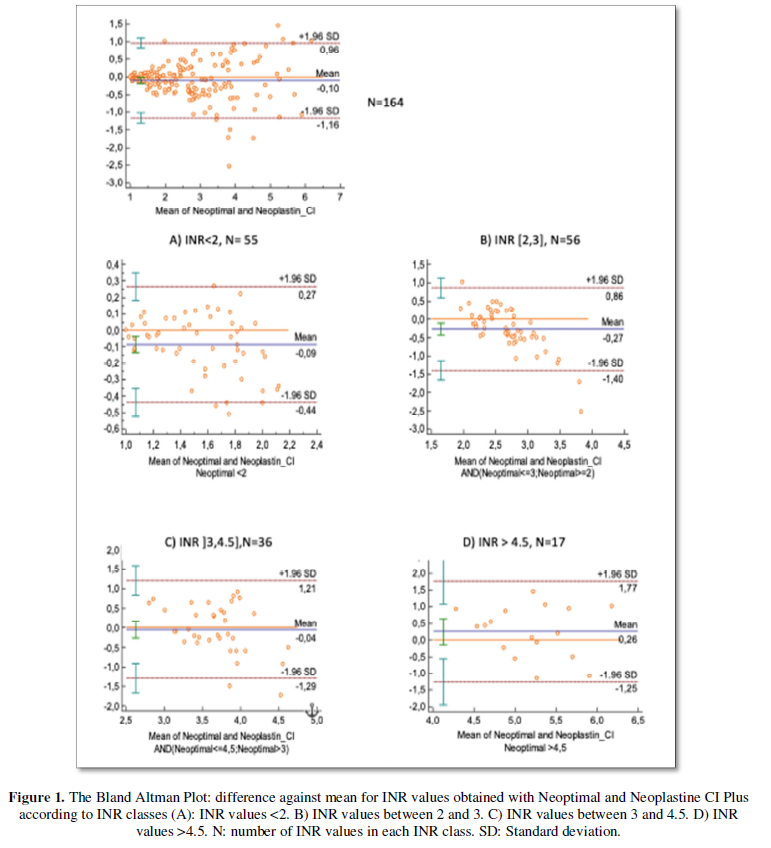
Seven plasmas did not clot. So, it was not possible de determine the INR values and have consequently been excluded from Bland Altman Plot. According to Bland Altman Plot (Figure 1), the mean difference of INR values obtained with Neoptimal and Neoplastine CI plus was -0.1 which means that INR values obtained with Neoptimal were in average 0.1 lower than those obtained with Neoplastin CI plus. The agreement limits were (-1.16, 0.96). Cohen’s Kappa coefficient was 0.76.
For "therapeutic" INR values (between 2 and 4.5), the mean difference was at most 0.26. The limits of agreement were respectively (-1.4; 0.86) for INR values between 2 and 3, and (-1.29; 1.21) for INR values between 3 and 4.5. When considering agreement limits between -1SD and 1SD, their values drop to at most 0.7.

Neoptimal vs Innovin
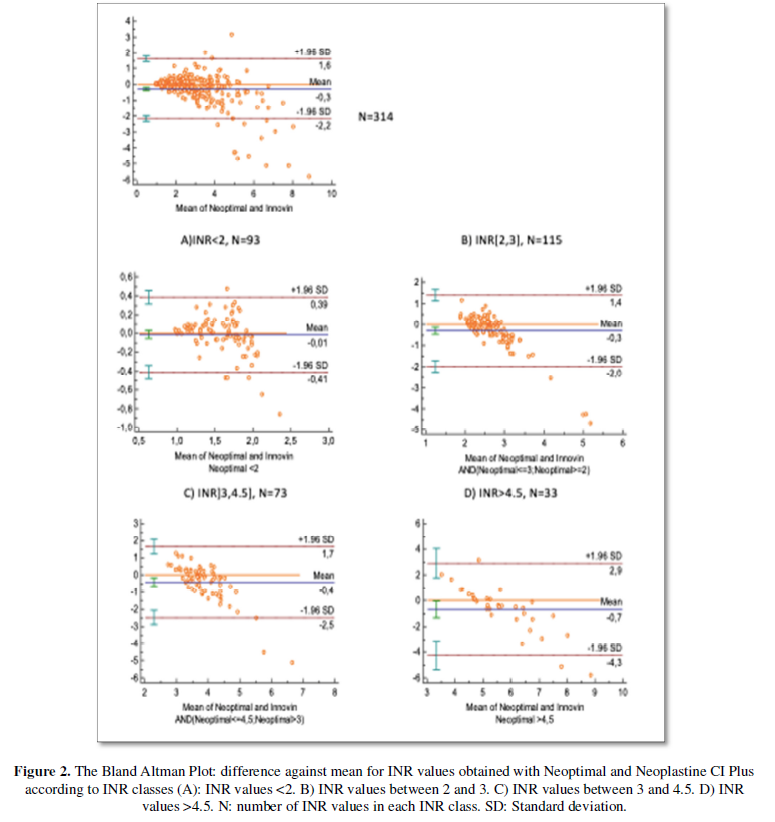
Seventeen INR values were non-coagulating and have been excluded from Bland Altman Plot. According to Bland Altman plot (Figure 2), the mean difference between INR values obtained with Neoptimal and Innovinwas -0.3.INR values with Neoptimal were in average lower than those obtained with Innovin. The limits of agreement were (-2.3, 1.6). Cohen’s kappa coefficient was 0.76.
For INR values between 2 and 3, the mean difference was -0.3 (-2; 1.4). For INR values between 3 and 4.5, the mean difference was -0.4 (-2.5; 1.7). When calculating agreement limits between -1SD and 1SD, the values were respectively (-1; 0.7) and (-1.25; 0.85).

DISCUSSION
To know if changing thromboplastin/instrument combination affects the follow up of patients on anti-vitamin K treatment, a study comparing the agreement between three reagent/instrument combinations largely used in laboratory routine was assessed. The three selected thromboplastins had ISI value close to 1. The collected samples were chosen to give a wide range of INR values.
Overall, there was a good agreement between INR results inferior to 2. The agreement was increasingly poorer with longer PT times. Besides, the discrepancy increased when both reagent and instrument vary.
Neoptimal vs Neoplastine CI plus
Cohen’s Kappa coefficient was high enough to consider that the agreement between the two reagents is substantial. Besides, the mean difference was -0.1 which is inferior to the recommended analytical bias (
Neoptimal vs Innovin
According to Cohen’s Kappa coefficient, the agreement between these two reagents was also substantial. The difference in INR values between these two reagents was -0.3 (-2.3; 1.6). A difference up to 2.3 is not clinically acceptable. This result was more evident for INR values between 2 and 4.5. Moreover, the limits of agreement was high enough to prompt an adjustment in anticoagulant dosage. Even for agreement limits between -1SD and 1SD, the values were still unsatisfactory with a difference in INR value up to 1.25.
This supports the inconsistency of INR values between laboratories which has been reported in previous studies [2,3]. The INR does in fact depend on different factors such as the value of mean normal PT determined by the laboratory, the exact value of ISI determined by the manufacturer, the variability of ISI between combinations and a significant part of systematic errors in PT which is influenced by local conditions [1,9]. It also depends on the type of the thromboplastin (recombinant or derived from animal tissue) as recombinant thromboplastin is known to be more sensitive and consequently associated to large variability in INR results [10]. The basis of the ISI principle has itself been criticized [7]. All these factors could explain the variability between different thromboplastins as shown in our study. The use of calibration with lyophilized calibrant plasmas has been proposed to reduce this variability inter laboratory [11]. This has also been shown in our previous study [4] when comparing Thromborel S reagent on SYSMEX 2100i and Innovin on SYSMEX 2100i using calculated INR and direct INR. The agreement was better when using the direct INR. Another alternative would be the use of Owren method for PT determination as it was reported that this method provided a bias less important and much less variable than the Quick method [7].
In conclusion, even if ISI is specific to reagent/instrument combination, it failed to generate the same INR which raises the concern about the reliability of INR concept. A better approach to avoid INR discrepancies would be that each laboratory adopts one single reagent/instrument combination to monitor patients on antivitamin K treatment.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
- Besselaar AMHPVD, Poller L, Tripodi A, Attermann J (2004) Definition of the International Normalized Ratio (INR) ans its consequences for thecalibration procedure of thromboplastin preparations: A rebuttal (multiple letters) [9]. J Thromb Haemost 2(8): 1490-1491.
- Ng VL, Levin J, Corash L, Gottfried EL (1993) Failure of the International Normalized Ratio to generate consistent results within a local medical community. American J of Clinical Pathology 99(6): 689-694.
- Swaim WR (1993) Prothrombin time reporting and the International Normalized Ratio system. Improvements are needed. Am J Clin Pathol 99(6): 653-655.
- Baccouche H, Chakroun A, Zoghlami A, Mahjoub S, Romdhane NB (2017) The international normalizedratio (INR): What reagent, what instrument? The assessment of the agreement between INR values according to differentreagent/instrument combinations. J Clin Pharm Ther 43(1): 52-58.
- Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1(8476): 307-10.
- Fuhrman C, Chouaid C (2004) Concordance of two variables: numerical approaches. Rev Mal Respir 21:123-125.
- Horsti J, Uppa H, Vilpo AJ (2005) Poor agreement amongprothrombin time international normalized ratio methods: comparison of seven commercial reagents. Clin Chem 51(3): 553-560.
- Lassen JF, Brandslund I, Antonsen S (1995) International normalized ratio for prothrombin times in patients taking oral anticoagulants: Critical difference and probability of significant change in consecutive measurements. Clin Chem 41(3): 444-447.
- Goguel AF, Houbouyan LL (1998) Standardisation de I'INR par une procedure utilisant des plasmas calibrés: l'expérience nationale française. Revue Française des Laboratoires 1998: 33-39.
- Gardiner C, Kohama K, Patel I, Lane P, Dwyer S, et al. (2017) Aperformance evaluation of a novel human recombinant tissuefactor prothrombin time reagent (Revohem™ PT). Int J Lab Hemtol 39(5): 532-538.
- Chantarangkul V, Tripodi A, Cesana BM, Mannucci PM (1999) Calibration of local systems with lyophilized calibrant plasmas improves the interlaboratory variability of the INR in the Italian external quality assessment scheme. Thromb Haemost 82(6): 1621-1626.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Alzheimers and Parkinsons Disease
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Allergy Research (ISSN:2642-326X)



