274
Views & Citations10
Likes & Shares
Bovine cysticercosis has a worldwide distribution and is most common in Africa, where poorly educated populations and low access to safe tapeworms favor the spread of Taenia saginata. In some countries, such as Kenya, 80% of cattle are said to be infected. The disease has little impact on animal health but is socially and economically important as it is a human and animal infection. The meat must often be condemned and costly extermination measures are necessary. The preferred host of T. saginata cysticerci is cattle, especially young animals, as older animals are more resistant to infection [6]. Livestock can act as an intermediate host for tapeworms in humans and other animals. Larval tapeworms (metacestodes) develop into fluid-filled cysts, each located in a typical location in the body. They act as space-occupying lesions and cause condemnation during meat inspections. Cattle around the world can carry the metacestode of Taenia saginata (the human tapeworm), also known as cysticercosis bovis, in their striate musculature [4].
Taenia saginata gestational proglottids are passed in the feces or removed from the anus; eggs are excreted through the proglottids; cattle are infected by ingesting eggs during feeding. While the larval stage can be found in cattle of any age, live cysts are mostly found in cattle less than two years old. The presence of the parasite in the muscles of cattle does not cause clinical symptoms unless the infection in the animal is very severe and involves the myocardium, but as it is a zoonotic infection its presence may cause losses. important to the beef industry, particularly in developing countries [7].
Human ingestion of cysts from raw or unprocessed beef, the parasite develops into the adult stage and completes its life cycle in the small intestine. After humans ingest cysts in raw or undercooked beef, it takes about two months for adult worms to develop in the intestine. In Ethiopia, T.saginata is so common that infected people treat themselves without even seeing a doctor [6].
Bovine cysticercosis occurs independently of clinical signs under natural conditions; however, experimentally infected calves developed severe myocarditis and heart failure, which were associated with the development of cardiac cysticercosis. In men, adult parasites can cause diarrhea and hunger, but the infection is usually asymptomatic [5]. There are currently no approved drugs effective in killing all cysticerci in muscle; praziquantel appears to be an effective drug of choice in experimental settings [6].
In developed countries, control of bovine cysticercosis depends on high standards of human hygiene, thorough cooking of meat to 570°C, mandatory meat inspection, and freezing of any affected carcass. cysticercosis at -10°C for at least 10 days of common practice, which is sufficient to kill cysticerci, although this process reduces the economic value of the meat. In agricultural practice, the use of human sludge as fertilizer should be restricted to arable land or land where livestock will not graze for at least 2 years. In developing countries, where similar measures are necessary but not always economically feasible, the most useful steps currently appear to be educating communities about hygiene and thorough cooking of meat [8].
Therefore, the primary objective of this study was to determine the prevalence and characterize the viability of cysticercus bovis at the Shone Municipal Slaughterhouse.
MATERIALS AND METHODS
Study Area Description
Shone is located in the Hadiya Zone of Southern Ethiopia, 345 kilometers from Addis Ababa. In terms of terrain, the altitude is 1650-2050 meters above mean sea level, the annual average temperature is 18%, and the relative humidity is 65%. The average temperature is 11 to 27°C, with few climatic fluctuations. There are two ecological zones in the entire district, 100 of which are the central line. According to Shone Town administrative agricultural statistics, there are 93,040 cattle, 15,457 sheep, 19,123 goats, 8,340 donkeys, 428 mules, 52 chickens, and 76,747 chickens in stock in the county. The production system throughout the region is of a mixed type. Geographically, the map of the study area was shown below (Figure 1).
Study animals
Study animals consisted of cattle randomly selected from municipal abattoirs in Shone; these animals were brought slaughter at various cattle markets around the Shone town authority. The animal breeds used in this study were almost all native breeds, some of which were hybrids.
Study design and research methods
The sample size required for the study was determined by calculating the sample size using the formula given in [17], as approximate past prevalence values are unknown. Therefore, an expected prevalence of 50% was considered and an ideal absolute precision (d) of 5% (0.05) with a confidence level of 95% was used in this study. Therefore, the calculated sample size is 384.
Ante-mortem inspection and post-mortem inspection
During an antemortem inspection of animals, details are recorded on the species, breed, sex, age, and origin of the animal. Age was estimated based on tooth type, and age was divided into young (<2 years), adult (2-5 years), and elderly (≥5 years). During the autopsy, the organs are systematically examined, namely the shoulder, the masseter muscle, the tongue, the heart, the liver, the lungs, and the diaphragm, applying the routine meat examination procedures, including visualization and palpation of organs and muscles, examination Presence of Cysticercus bovis cysts. If single or multiple bovine cysticercosis are found, further incisions are made in each organ and the number of cysts per organ per animal is registered.
Cyst Viability Testing
All positive samples are shipped to Shone Laboratory to confirm cyst viability. Incubated cysts in ox bile at 37°C for 1-2 h using 40% ox bile solution diluted in saline. After that, examine the scolex under a stereomicroscope, if the scolex is evaginated during the latent period, the cyst is considered viable and also checked for absence of hooks on the rostellum of the evaginated cyst [3,10].
Data management and analysis
Post-mortem meat inspection data collected were recorded and entered into a Microsoft Excel 2007 spreadsheet and statistically analyzed using STATA version 11.0 [11]. The results of the analyzes were evaluated using Pearson's chi-square (x2) to look for significant differences in the contribution of the variable to the incidence of cysticercosis.
RESULTS
Prevalence study
Bovine cysticercosis had been identified in 28 out of the 384 animals examined in the Shone municipal abattoir, with a prevalence of 7.29% overall. The incidence of the disease did not differ significantly between animals based on sex, breed, or origin (p > 0.05). The highest prevalence was observed in cattle from Shone (8.24%), followed by East Badawacho (7.95%), Lenda (7.23%), Jarso (5.56%), and Korga (0.00%) (Table 1).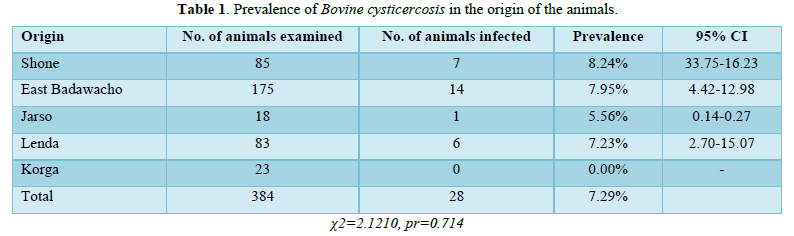
The study examined a total of 384 animals, out of which 28 were infected with C. bovis, resulting in an overall prevalence of 7.29%. This result shows that the prevalence of C. bovis was higher in female cattle (10.71%) compared to male cattle (6.7%). This difference in prevalence between sexes was statistically significant, as the 95% confidence intervals (CI) for the two groups did not overlap. The higher prevalence of C. bovis in female cattle could be attributed to factors such as age, breed, and management practices. Additionally, female cattle may be more likely to be kept for longer periods, increasing their exposure to infection (Table 2).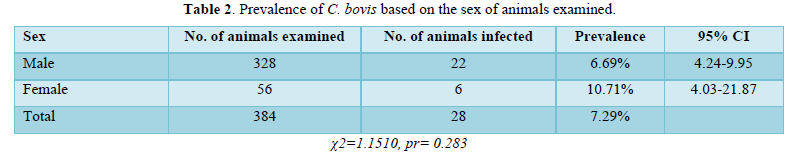
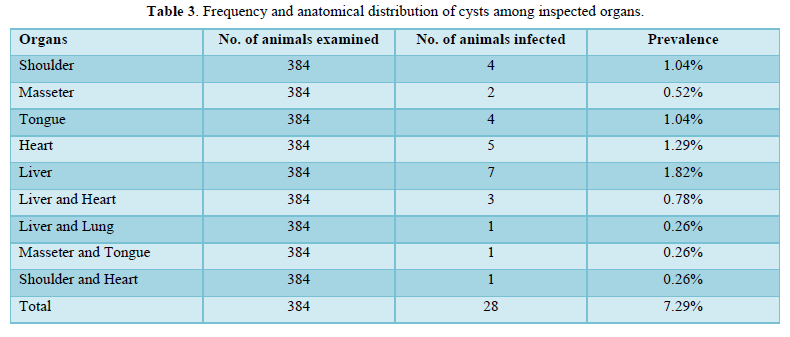
The liver, heart, and tongue had the greatest concentrations of cysticercus bovis among the affected organs and muscles. The fewest cysts were found in the lungs. The remaining 6 (21.43%) of the infected animals had cysts in numerous organs, although the majority of the affected animals, 22 (78.57%), had cysts in just one organ or tissue (Table 3).
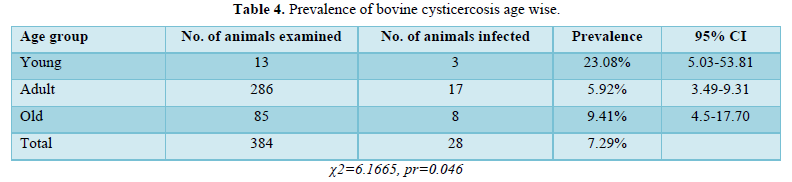
A frequency of 5.92% was identified among the 287 adult animals investigated, of which 17 were identified to have cysticercus bovis infections. With 8 infected out of the 85 aged animals, the frequency was 9.41%. Three (23.88%) of the 13 young animals evaluated had C. bovis infections. Age and infection rates were shown to be significantly correlated (p<0.05) in the analysis (Table 4).
Among the 378 local breed animals examined, 27 were found to be infected, resulting in a prevalence of 7.09%. Only 1 out of the 6 cross-breed animals examined was infected, resulting in a prevalence of 25.00% (Table 5). Out of 126 cysts collected from 28 animals over the course of the study. 50 (39.7%) of the total cysts were degenerated cysts, whereas 76 (60.30%) were viable cysts (Table 6).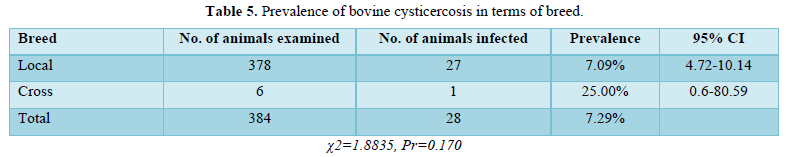
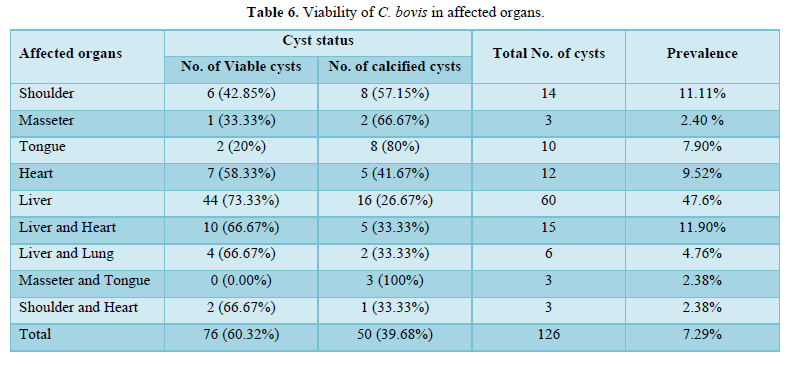
During this study period, a total of 126 cysts were collected from 28 animals at Shone municipal abattoir, of the total cysts collected 76 (60.32%) were viable whereas 50 (39.68%) degenerated cysts (Table 6).
DISCUSSION
In this study, 28 of 384 animals examined at Shone Municipal Abattoir were identified as positive for bovine cysticercosis. Therefore, the overall prevalence rate was 7.29%. The prevalence of the results of this finding is broadly in line with those of Addis Ababa (10) (7.5%) and (7).01%) in Mekele. If this result is compared to the reports of other workers such as (16) (3.11%) and (6) (4.9%), the incidence is higher in central Ethiopia and Gondar, respectively. (1) (26.25% of Awassa and (9) (30%) reported a higher prevalence of C. bovis in all of Ethiopia. Factors such as sample size and practical limitations in the number of incisions allowed per organ during examination mean that many infections can go undetected. The owner did not allow multiple incisions for detailed examination because mutilation would reduce the marketability of the corpse and introduce contamination.
There was no correlation in this study (P > 0.05) Relationship between sex, origin, breed, and prevalence of cysticercosis. Analysis based on sex was not statistically significant, with a prevalence of 22 males (6.7%) and 6 females (10.7%). This is because both sexes graze on the same pastures and therefore face similar challenges to T. saginated eggs. In terms of origin, the majority of animals destined for slaughter at the study abattoirs came from stock breeders' associations in Shone, East Badawacho, Lenda, Korga, and Jarso.
The prevalence of cysticercus bovis in young (23.08%) was higher than in adults (5.92%) and old age group (9.41%), the difference between age groups was statistically significant (P<0.05). This marked change in the prevalence of C. bovis may be due to age-related immunity; the re-stimulation of the animal's immunity after a persistent invasion by onchospheres could explain the development of more cysticerci from invading onchospheres [12]. In this study, cysticerci were distributed throughout the organs examined, and the muscles of the liver, heart, tongue, and shoulders were the most affected organs, with the highest number of cysts, but the least in the lungs. Therefore, the most affected organ with the highest number of cysts was the liver, which is consistent with the report [13] indicating that the liver was the most severely infected organ. However, Tembo [14] have shown that the heart is the most seriously infected organ.
Cyst viability testing showed that the liver had the highest number of viable cysts, followed by the heart, tongue, and shoulder muscles respectively. Changes in anatomical distribution depend on many factors, such as hemodynamics and the daily activity of the animal. All geographic and environmental factors that affect the animal's hemodynamics can affect the distribution of cancerous spheres and their preferred location during meat inspection [3].
CONCLUSIONS AND RECOMMENDATIONS
The results of the slaughterhouse survey show that the prevalence of bovine cysticercosis is 7.29%. Although the prevalence rate is not very high, infection with C. bovis Livestock diseases in cattle causes economic losses due to organ/tissue discard, while taeniais cause public health problems and economic losses due to drug costs. Risk factors for bovine cysticercosis and its importance to public health are due to lack of adequate meat inspection, inherited traditions of eating raw or undercooked meat, lack of public awareness of pasture contamination by human excreta, and poor sanitation and sanitation infrastructure. This often leads to contamination of the environment, especially pastures by Taenia bovis eggs. Based on the above conclusions, the following recommendations are made:
- Pay attention to routine meat inspection and further investigate the incidence and public health significance of bovine cysticercosis.
- People should be informed about the dangers of eating raw or undercooked meat and the risk of bovine cysticercosis.
- Educate people on toilet use and improve personal and environmental hygiene.
- Bovine cysts are a cause of economic loss and danger to public health and organizations such as health, agricultural and educational institutions should be engaged to provide appropriate administrative, technical, and financial support for control and eradication successful.
- Cook meat thoroughly to kill larval cysts if present, another important aspect is to practice good hygiene, e.g. washing hands after using the toilet. This will prevent the spread of tiny eggs and other contaminants.
ETHICAL APPROVAL AND CONSENT FOR PARTICIPATION
Ethical approval for this study was granted by the Research Ethics Committee of the University. Adherence to advanced veterinary medicine guidelines and explanation of research purpose.
- CSA (Central Statistical Agency) (2019) Federal Democratic Republic of Ethiopia, Agricultural Sample Enumeration Abstract.
- OIE (Organization International des Epizootics) (2000) Manual of Standards for Diagnostic Tests and Vaccines. Cysticercosis pp: 423-428.
- Gracey J, Collins D, Huey R (1999) Meat Hygiene, 10th WB. Saunders, Co. London. pp: 669-678.
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2006) Veterinary Medicine, 10th A Text Book of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. Saunders Elsevier, London pp: 1582-1583.
- Urquhart GM, Armour J, Duncan JL, Dunn AM, Jennings FW (1996) Veterinary Parasitology, 2nd edn, Longman and Scientific. UK.
- Smyth JD (1994) Introduction to Animal Parasitology, 3rd Cambridge University Press pp: 326-348.
- Dwight D, Randycarllynn C, Mark LE, Ana A (2003) Geogis’ Parasitology for Veterinarians 8th Elsevier, USA.
- Taylor M, Coop R, Wall R (2007) Cestodes: In Veterinary Parasitology 3rd Blackwell Publishing Pty Ltd, Garsington Road Oxford Australia pp: 121-123.
- Thrusfield M (2005) Veterinary Epidemiology, 3rd Blackwell Sc, Edinburgh pp: 232-233.
- WHO (World Health Organization) (1983) Guidelines for Surveillance, Prevention, and Control of taeniasis/cysticercosis. In: Gemmell, M., Z. Matyas, Z. Pawlowski, and E. J. L. Soulsby (Eds), WHO, Geneva.
- Stata Corp (2001) Stata Statistical Software Release 11.0, Lake-way Drive, College Station, Texas.
- Wanzala W, Abuje OJ, Kang A, Ethe E, Zessin K, et al. (2003) Analysis of Post-mortem Diagnosis of Bovine cysticercosis in Kenya Cattle. Online J Vet Res 1: 28-31.
- Umer A (2009). Prevalence of Bovine cysticercosis at Dire Dawa Municipal Abattoir, DVM Thesis Faculty of Veterinary Medicine, Haramaya University, Ethiopia.
- Tembo A (2001) Epidemiology of saginata Taeniasis and Cysticercosis in Three Selected Agro Climatic Zones in Central Ethiopia. MSc Thesis, Faculty of Veterinary Medicine, Addis Ababa University & Free University of Berlin, Debre Zeit, Ethiopia.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Food and Nutrition-Current Research (ISSN:2638-1095)


