43691
Views & Citations42691
Likes & Shares
Donkey meat is a delicacy and the milk are believed to treat whooping cough in northwestern Kenya and Southern part of Ethiopia [6]. The oldest feral horse or Kundudo which was the only wild horse left in East Africa is found in Ethiopia. One of the oldest recorded breeds, the Oromo bloodlines come from Ethiopia and later spread along the coast of the Red Sea. They were first imported into England in 1861, where they quickly became prized for several of their unique characteristic [7]. The highest Equine population in Ethiopia is found in the Oromia region mainly of the Arsi-Bale highlands [8]. Equines mostly has intimate association with man and have huge role through their involvement in different social and economic activities. In Ethiopia they have been indicated as animals of burden as pack animals for long period of time and still render valuable services throughout the country particularly in areas where modern means of transportation are absent, unaffordable or inaccessible [9].
Parasitic infection is a major cause of illness of mules and donkeys, even though they succumb to a variety of diseases and a number of other unhealthy circumstances. Among these, [10] pneumonia is caused by parasites in the horse. Dictyocaulus arnfieldi is the true lungworm found in the horses, belonging to the super family of Trichostrongyloidea. It has been reported from various parts of the world [11-14]. Lungworms are found throughout every continent where equines are reared particularly common in countries with temperate environment and in the highlands of tropical and subtropical countries. Dictyocaulidae is known as endemic in East African countries such as Ethiopia, Kenya, and Tanzania and South Africa [15].
Dictyocaulus urnfield is one of lungworm species that affecting domestic equines and zebras [16]. Off the three equine species Donkeys and Mules are the natural hosts for lungworms. The condition in horses is usually found in those horses that have been living in the company or compound with donkeys and mules [17]. Donkeys have been found to be the major host and most important reservoir for Dictyocaulus urnfield. They are considered to act as the source of infection; horses play only an ancillary role and become infected after being grazed the same pasture with donkeys [18-20]. Unfortunately, they are rarely given any veterinary attention, they receive no feed supplements and their owners are often unaware of improved saddling techniques that would reduce the back sores their animals suffer from [21].
In Ethiopian even though there is large number of an equine population and the importance of equines in the economic activities in the country is increasing, very little research relating to Dictyocaulus urnfield (equine lungworm) has been conducted. Apart from few studies in other parts of Ethiopia, there has not been any previous information on equine the lungworm in the Lemmu bilbilo district.
Therefore, the objectives of this study were designed to estimate the current status of Dictyocaulus infestation and to identify the circulating species and associated risk factors for Dictyocaulus infestation.
Related Literature Review
Definition and Etiology of lungworm
A parasitic nematode worm of the super family of Trichostrongyloidea, order Strongylidae that infest the lungs of vertebrates are called Lungworms. The taxonomy of this parasite is belonging to the kingdom Animalia, phylum Nematode, class Secernentea, family Dictyocaulidae, genus Dictyocaulus and species of Dictyocaulus urnfield [22]. Dictyocaulus urnfieldi is the particular lungworm species that affect donkeys, horses, and mules [16]. In endemic areas it is pathogenic in horses and considered as a relatively well-adopted parasite of donkeys [23].
Morphology of Dictyocaulus arnfieldi
Adult Dictyocaulus worms are slender, medium-sized roundworms, up to 8 centimeters long. Females are about one-third longer than males. They have a whitish to grayish color. The body of these lungworms is covered with a cuticle, which is flexible but rather tough. The worms have a tubular digestive system with two openings, which is the mouth and the anus. They no excretory organs and no circulatory system but have a nervous system. The female Dictyocaulus arnfieldi ovaries are large and the uteri end in an opening called the vulva. Males have a copulatory bursa with two short and thick spicules for attaching to the female during copulation. An ovoid shaped egg of Dictyocaulus arnfieldi are approximately 5.4 × 103µm and contain a well-developed L1 larva [24]. Lungworm larvae are slender and 25 - 70 mm long. The Dictyocaulus arnfieldi larvae resemble those of Dictyocaulus viviparous but the tail ends in a small spine [25].
Epidemiology of Dictyocaulus worm
The epidemiology of lungworm infestation is largely concerned with factors determining the number of intensive larvae on the pasture and the rate at which they accumulate. The third stage larvae or L3 are long living in damp and cool surroundings. Summer season give rise to heavier burdens of lungworm infestation and followed by autumn and spring season of the year. In most instances, horses acquire this disease when pastured with donkeys and do not usually transmit the disease to other horses [26].
Under optimum condition, the larvae may survive in the pasture for twelve months. The larvae are quite resistant to cold temperature although it generally delay their maturations. They can withstand cool season especially autumn and early winter up to temperature of 4-5°C [27]. The larvae stages of these lungworms tolerate and prefer low temperatures, which leads to the occurrence of verminous pneumonia outbreak [15]. The natural host of the parasite is donkey, and comparably, higher numbers of parasites can infest the lungs of this host without clinical signs. Donkeys and mules can act as a reservoir for horses [19]. Pilobolus fungi may play a role in the dissemination of Dictyocaulus arnfieldi larvae from stool, as Dictyocaulus viviparous. Dictyocaulus Arnfieldi is found globally, particularly in areas with heavy rainfall [28].
Life Cycle
The detailed life cycle is not fully known, but is considered similar to that of bovine lungworm, Dictyocaulus viviparous except in the following respect. The adult worms are most often found in the small bronchi and their eggs which contain the first stage larvae (L1), hatch soon after being passed in the equine stools [28]. Lungworms have a direct life cycle that means there are no intermediate hosts involved. Adult females lay eggs in the respiratory tracts of infected hosts. These eggs with the aid of respiratory secretions transported to the pharynx. From the pharynx, these eggs are coughed out directly to the outside or into the mouth to be swallowed back. Those eggs that are swallowed from the mouth release the first stage or L1 larvae in the gut, which are shed in the equine feces [24].
In the environment, first stage larvae develop to infective third stage larvae in about seven days. The L3 larvae show a low motility and remain close to the droppings. Animals become infected mainly while grazing, but contaminated hay or bedding can cause indoors infection. After swallowed, in the host's gut L3 larvae penetrate into the gut's wall and reach the lymphatic system where the molt to fourth stage larvae (L4). They reach the heart and are pumped to the lungs through the thoracic duct and the jugular vein. In the lungs the larvae are stopped movement for some period in the lung capillaries, then cross the capillaries wall and reach the bronchioles, bronchi or the trachea where they complete development to adult lungworms. The pre-patent period lasts about one months. However, larvae in the lungs may become arrested for up to 300 days [24] (Figure 1).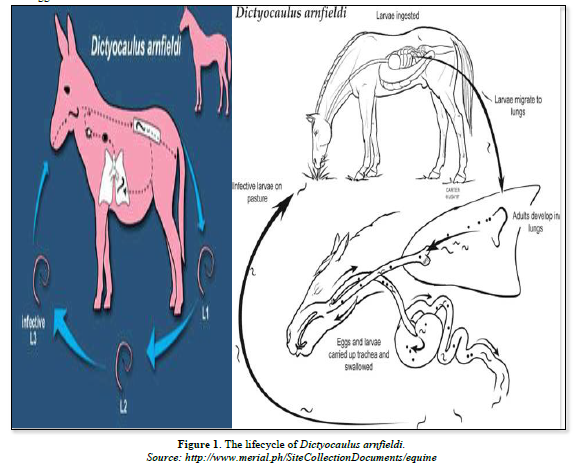
Pathogenesis
The pathogenic effects of lungworm influenced by different factors such as the location of parasite within the respiratory tract, the number of infective larvae ingested, the animal immune status, the nutritional status, seasonal variation and the host age [25,29]. An inflammatory response, which may block small bronchi and bronchioles with inflammatory exudates resulted due to migration of Larvae through the alveoli and bronchioles. The bronchi contain fluid and immature, latter adult worms and the exudates they produce. Secondary bacterial pneumonia and concurrent viral infections are of the complication of lungworm infestation [30]. The major pathologic changes that results from primary infection may be divided into three stages. These are the pre-patent stages, where blockage of small bronchi and bronchioles by eosinophilic exudates produced in response to the developing and migrating larvae. The patent stage, when adult lungworms leads to the occurrence bronchitis and development of primary pneumonia. The post patent phase is when adult lungworms are expelled and majority of animals recover gradually. The pathological changes seen in the lungs during necropsy finding are atelectasis, emphysema, petechial hemorrhage and lung consolidation [31].
Clinical Signs
Despite the prevalence of patent D. arnfieldi infestation in donkeys, overt clinical signs are rarely seen; however, on close examination slight hyperpnoea and harsh lung sounds may be detected. This absence of significant clinical abnormality may be partly a reflection of the fact that donkeys are rarely required to perform sustained exercise. Infection is much less prevalent in horses. However, patent infections may develop in foals and these are not usually associated with clinical signs. In older horses, infections rarely become patent but are often associated with persistent coughing and an increased respiratory rate [28]. Donkeys usually show no clinical signs of the disease and can be silent carriers which act as shedders of this parasite. In horse’s lungworm infestation can show the clinical signs [22].
Diagnosis
Diagnosis is depending on clinical signs, epidemiology, presence of first-stage larvae in feces, and necropsy of animals in the same herd or flock. Bronchoscope and radiography may be helpful. Larvae are not found in the feces of animals in the pre-patent or post-patent phases and usually not in the reinjection phenomenon. ELISA (Enzyme Linked Immuno Sorbent Assay) tests are available in some laboratories. Bronchial lavage can reveal Dictyocaulus arnfieldi infections in horses [32]. Verminous pneumonia is easily confused clinically with bacterial bronchopneumonia, with acute and chronic interstitial pneumonia, and with viral pneumonia. The lungworms disease usually occurs in outbreak form in summer and autumn season of the year [26]. There are various diagnostic methods used to detect lungworms infestation as described below in details.
Clinical Diagnosis
Typical signs and symptoms include: heavy coughing, accelerated breathing, dyspnea (difficulty in breathing), anorexia, lose weight and nasal discharge. Severe infections can also cause pneumonia, emphysema and pulmonary edema. Adult livestock usually develops resistance and if re-infected may not show clinical signs but continue shedding larvae that contaminate the environment [24].
Fecal Examination
A conventional method for recovering larvae is a modified Biermann technique in which 5-10 grams fecal samples are wrapped in tissue paper or cheesecloth and suspended or placed in water contained in a beaker. After decanting the water slowly, the water at the bottom of the beaker is examined for larvae after 4 h; in heavy infections, larvae may be present within 30 minutes. Bronchial lavage can reveal Dictyocaulus arnfieldi infections in horses [32].
Serological Diagnosis
Enzyme Linked Immuno Sorbent Assay (ELISA) test can demonstrate antibodies from five weeks after the animals have been exposed and it may be useful in identifying infected animals when heavy burdens of worms do not generate and larvae in the feces. This time need to perform an ELISA depends on the availability of antigen-coated microstate-plates. If such plates can be provided, the result can be obtained within four hours after the serum has been prepared. If not, plates have to be coated with antigen for up to 16 h [33].
Necropsy Findings
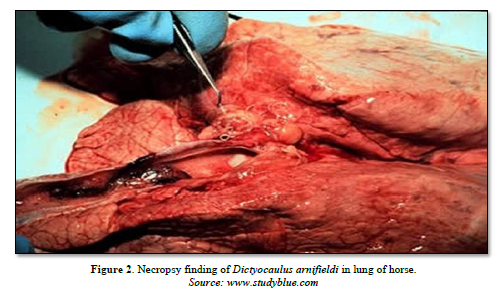
The morphological change in the lungs include wide spread areas of collapsed tissue of dark pink color, hemorrhagic bronchitis with much fluid filling all the air passed and enlargement of the regional lymph nodes. Histological, the characteristic lesions are edema, eosinophilic infiltration, debris and larvae in the bronchioles and alveoli. The most obvious lesions at necropsy are discrete patches of over inflation. The bronchial epithelium is hyperplasic and heavily infiltrated by inflammatory cells, particularly eosinophils [34] (Figure 2).
Differential Diagnosis
On a clinical basis, bacterial pneumonia is considered as the first tentative diagnosis. Other probable tentative diagnoses are considered such as chronic hypersensitivity pneumonitis, chronic obstructive pulmonary disease, fungal pneumonia, immune mediated interstitial or vascular disease and unusual drug reactions as well as foreign body in the trachea [35].
Control and Preventions
Routine deworming of horses and donkeys may help to prevent cross infection when kept together. Pastures in which donkeys are grazing may be infected with lungworm larvae. As a result, horses and donkeys should not be grazed together [22]. Reducing pasture contamination with infective or third stage larvae is a key preventative measure that can be achieved largely with adequate practice of a management measures. Rotational grazing with a change interval of 4 days and keeping the paddocks empty for at least one month and 10 days significantly decrease pasture contamination. This is because larvae are susceptible to dryness and will not survive greater than one months on pasture. Obviously, by very moist weather or where pastures are almost permanently moist survival may be longer. Alternate grazing with sheep may be considered since Dictyocaulus species are quite host-specific.
The reduction of specific lungworm burden reveals the absence of the specific host in the area longer period of time. However, this may not be advisable in places infected with gastrointestinal roundworms that affects cattle and sheep or horses. For their first grazing period, it is advisable that young stock does not share the same pastures with older stock which have been exposed to infected grounds, and can shed larvae. It must also be avoided that young stock uses pastures already used by older stock during the same season. It must also be considered that heavy rains and flooding can disseminate third stage or L3 larvae inside a pasture or from one pasture to the nearer ones. Keeping the pastures as dry as possible and keeping livestock away from places excessively humid are additional crucial technique to reduce the exposure of livestock to infective larvae. In endemic regions preventative strategies focus on deworming of young stock which is often recommended just prior to their first grazing season, followed by additional deworming treatments depending on the infestation burden of the pastures and the residual effect of the administered anthelmintic [24] (Table 1).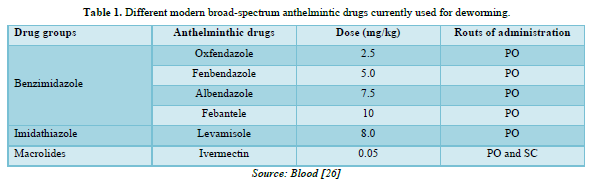
Economic Importance of the Disease
Even though equines play a significant role as pack animals in the economy of the country, the government overlook it and focused on other livestock. Also, the development programmes aids agencies are aimed towards in meat, milk, and egg and wool production. Equines have been completely neglected or omitted from the pastoral livestock programmes. This is because of the contribution of equines power in the agricultural system and their role in the productions not yet well recognized and magnified [36].
The vitality and well-being of horses of all age are thread by a variety of internal parasites and the use of control ensures and the best performance [37]. Internal parasites are one of the greatest limiting factors to successful horse rising throughout the world. All horses at pasture become infected and suffer a wide range of harmful effects ranging from impaired development and performance to death despite the availability of large array of modern anthelmintic, parasite controls often fail to safeguard horse health. The main reason for these break downs are errors the choice of anthelmintic and in the time of treatment [38].
The status of Lungworm Infection in Ethiopia
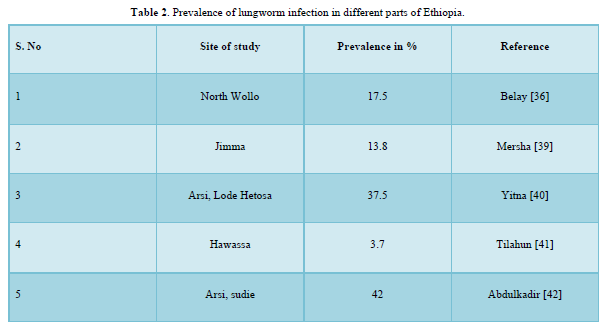
The concern of lungworm in Ethiopia is increasing and is now a major problem of equine in the central highlands of Ethiopia. However, there were little preliminary findings of lungworm infection, which were done by few researchers of the country (Table 2).
MATERIALS AND METHODS
Description of the Study Area
The present study was conducted from November 2016 to march 2017 in Lemu Bilbilo woreda located in the Arsi zone of the Oromia region. Bokoji town is the administrative center of woreda and has a latitude and longitude of 7°35′N39°10′E with an elevation of 2810 m. It is located 56 km south of Asella and 230 km southeast of Addis Ababa. According to woreda, Livestock and fishery resource development office (2012) the total area of the district is 814 km2 and sub divided in to 25 administrative units. The Altitude of the district ranges from 2500-4245m a.s.l. The ecological zone of the district is mainly high land with small portion of mid land while the climatic condition of the area is “weynadega”. The mean maximum and minimum temperature are 28°C and 10°C, respectively. The annual rainfall is 700-1658 mm with a bimodal rainfall occurring from March to April (short rainy season) and from July to October (long rainy season). The total number of equine populations of the district was 107052. Among these 57083were horse, 45045were donkey, and 4954 were mules. From this district, ten kebeles were randomly selected. Namely; Lemmu Dima, Tullu Negesso, Temegne Aware, Lemmu Jara Ciba Michael, Koma Kaka, Lemmu Mirti, Ululle Kara, and Koji Qubsa. The sample was collected properly from each kebeles and transported to Asella regional veterinary laboratory (Figure 3).
Study Design and Sampling Procedures
The study type was cross-sectional and it was a simple random sampling technique used to select individual study animals. The desired sample size was calculated according to the formula given by Thrusfield [43] at 50% of expected prevalence setting 95% of confidence interval and a P-value of less than 0.05 was taken as statistically significant.
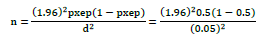
Where n: Sample size required; pxep: Expected prevalence; d2: Desired absolute precision at 95% Confidence interval.
The numbers of equines required for study at 95% of confidence interval, 5% absolute precision and expected prevalence of 50%. Therefore, the calculated sample size was 384.
Fecal Samples Collection and Examination
The study animals were randomly selected horses, donkeys and mules in Lemmu Bilbilo district, Arsi zone. Fecal samples were collected directly from the rectum using arm length gloves and transferred to screw-corked universal bottles that was labeled accordingly and soon brought from study area to Asella Regional Veterinary Laboratory. Information about the age, sex, body conditions, owner name and date of collection was recorded. Faecal samples were processed on the day of collection. Coprological examination was performed using a modified Biermann technique for the detection of Dictyocaulus arnfieldi first stage larvae (L1) following standard procedures described by Anne and Gray [44]; Charles and Robinson [45].
Age and Body Condition Estimation
The age of the selected animals was determined using the dentation method and by asking owner [46,47]. Animals were grouped into three age groups namely young (≤ 3 years) (n=77), adult (4-10 years) (n=195) and old (˃10 years) (n=112). Body Condition score was assessed subjectively using a scale from 1 (emaciated), 2 (thin), 3 (average), 4(fat) to 5 (very fat) [47]. The body condition of equines was categorized as poor, medium and good.
Data Managements and Statistical Analysis
The collected data during sampling and laboratory results were entered and stored in Microsoft Excel spreadsheet 2007.the data were transported to the SPSS software version 20.0 statistical tools. The data were thoroughly screened for errors and properly coded before subjecting to statistical analysis. Percentage were used to measure the prevalence of helminthes and chi-square (χ2) test was to measure association between prevalence of the helminthes and the breeds, age, and body conditions of animals were the applied. The confidence level was held at 95% and the results were considered significant at P<0.05.
RESULTS
The Overall Prevalence of Equine Lungworm and Associated Risk Factors
The overall prevalence of equine lungworm in the study area was 34.9% with the prevalence of 60.65%, 48.1% and 17.7% in donkeys, mules and horses respectively in which statically significance difference (P<0.05) observed between the three species. There was statically significance variation in age groups and body condition score whereas no statically significance variation in sex group (Table 3).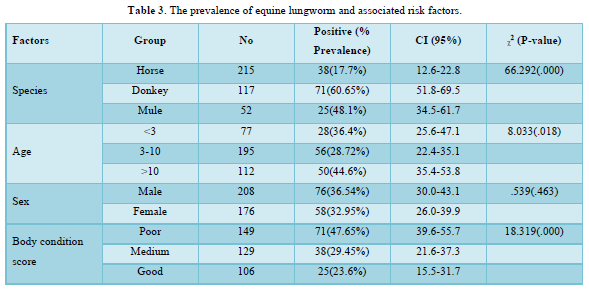
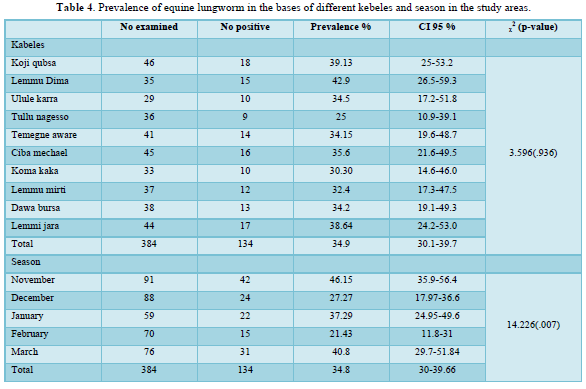
The present study result revealed that there was no statistically significant difference (p>0.05) observed in the kebeles of the study area (x2=3.596, P=0.936). Although the prevalence of lungworm in Lemmu dima kebele in this study was highest (42.15%) as compared to other kebeles, the prevalence of the disease was lowest (25%) in Tullu Negesso kebele as compared to other kebeles of the study area (Table 4).
DISCUSSIONS
The overall prevalence of lungworm infection in the study population was 34.9% (134/384). This overall prevalence is smaller than the findings of Yitna [40] and Abdulkadir [42] reported a prevalence of 37.5% and 42.7% in Arsi Lode Hetosa, and Arsi Sudie respectively. However, this study result is higher than the previous study results of Getahun [48]. Shiferaw [49], Adere and Tilahun [50] reported a prevalence of 20%, 23.2% and 11.2 at Bale, Wonchi and Jimma, respectively. This variability may be due to the differences in environmental conditions between the study area and or management practice of both the equines and pasture which may suites to the survival of the larvae of the lungworms.
In this study, relatively higher overall prevalence of Dictyocaulus arnifieldi was recorded in donkey (60.65%) than in mule (48.1%) and horses (17.7%). This was also true from the reports of Legese [51] who described a prevalence of 30.5-32.8% in Dira Dawa and Estern Ethiopia. Mersha [39] also reported similar finding that there was higher occurrence rate of Dictyocaulus arnifieldi in donkeys (35.29%) than in mules (29.26%) and in horses (4.26%). The observed higher prevalence in donkeys could be reservoir host [17], and attributed to the fact that less attention is given to these animals that is by far lower than their workload [21]. The present study report on the prevalence of Dictyocaulus arnifieldi in donkeys (60.68%) was found lower than the reports of Feseha [5], Hassan [52] and Andersen and Fogh [53]; which was 83 %, 70.5% and 87.5% in Ethiopia, Sudan and Denmark, respectively. On the other hand, lower prevalence of lungworm in donkeys reported by Ayaz [54] and Bakirci [11] as 0.6%-14.6% and 9.67% respectively.
The prevalence of Dictyocaulus arnifieldi infestation in mules (48.1%) was greater than previous finding of Esheta [55], who reported 20% prevalence of D. arnfieldi in and around Bahir Dar. However, higher prevalence (54%) of lungworm than the present study in mules was reported by Lyons [56] in Central Kentucky. In this study, lowest infection rate was observed in horses (17.7%). This result was higher than the findings of Yacob and Ashenafi [57], Yitna [40] who reported, 0.5% 9.37 in Arsi-Bale, and Lode Hetosa district respectively. These differences in prevalence might be due to differences in management systems, season, sample size and agro-ecology.
The age of animals was found as one risk factor for the variation (P=0.018) in the prevalence of lungworm infection. A prevalence of 36.4%, 28.7% and 44.6% were recorded in animals <3 years, 3-10 years and >10 years of age, respectively. The present result agreed with Tihitna [58] reported as 18%, 10.98% and 22.95% in young, adult and old age groups, respectively and Adere and Tilahun [50] reported as 20.6%, 12.6% and 5.6% in old age young and adult respectively which agree with this study. But the prevalence of lung worm was highest in the young when compared with old and adult according to the report of Abdulkadir [42] and Yitna [40] which is 50.9% and 49.15% respectively. The highest infection rates (44.64% and 36.37%) were recorded in old and young age groups, respectively. This might be related to the condition that older and younger animals are taught to have decreased immunity [53].
The body condition of animals was also classified as poor, medium and good body condition scores. The prevalence according to body condition score was 47.65%, 29.45% and 23.6% in poor, medium and good body condition scores of the animals respectively. The current study agrees with the result of Yitna [40] reported 57.89%, 43.72%, and 15.18% in poor, medium and good body condition of animals respectively. Similarly, Abdulkadir [42] and Adere and Tilahun [50] indicated [59.6% (poor), 41.46% (medium), and 21.3% (good)] and [31.5% (poor), 10.8% (medium) and 3.1% (good)] in body condition of animals respectively. In addition, in different species of equids (Donkeys, horses and mules), body condition score was considered as a risk factor and was statically significant (p<0.05).
Out of the selected ten kebeles in the district, the prevalence of Dictyocaulus arnifieldi in Lemmu dima (42.1%) was highest and lowest in Tullu Nagesso (25%) as compared to other selected kebeles of the study area although it was insignificant variation (x2=3.596, P=0.936,) as shown in Table 4. The highest and lowest prevalence in Lemmi Dima and Tullu Negeso respectively. This may be due to agro-ecological difference and the populations of donkey were different between the kebels. Concerning the seasonality of infection, in the current study it was found that the prevalence of infection was high in November (46.15%) and March (40.8%) than the rest of the study months. The environmental condition during these two months is relatively favorable due to the better rain availability while the rest months are not as they are relatively day. Hence, during this adverse climatic condition larvae of lungworm prefer to inhibit in the lung tissue and mature at the begging of the rainy season.
CONCLUSION AND RECOMMENDATIONS
The present study indicated that equine lungworm to be the major problem in the area being.
Highest in donkeys followed by mules and horses. Mostly the disease affects animals having poor and medium body condition as well as old and young due to inadequate development of the immune system in young and reduced immunity in old animals. Additionally, the grazing habit and seasonal variation of the area affect the status of the lungworm. The climatic condition of Lemmu Bilbilo, Arsi zone where rainfall is frequent and temperature is mild also favors the development and survival of infective larvae for most part of the years. Owing to the huge equines, population in the Lemmu Bilbilo woreda considerable contamination to the communal pasture grazing system could be the other factor, which favors the survival and development of the parasite larvae. Animals of different age and sex group usually graze on communal pasture facilitated easy transmission of this parasite. However, the problem due to lungworm of equines in the study area was given less attention because of its sub clinical nature. Hence, strategic treatment of equines should be undertaken based on sound and complete understanding on the epidemiology of lungworm of equines in the study district.
Therefore, based on the above conclusions, the following recommendations are forwarded:
- Strategic deworming using full dose broad-spectrum anthelmintic drugs and rotational grazing program should be implemented to reduce pasture contamination and infection of reservoir hosts especially in donkeys.
- Donkeys should graze in a separate pasture from horse and mule.
- The owner of equines should be gate awareness about how to improve the management system and health care of their equines.
- Further study should be initiated for assessment of risk factor and status of the disease.
- FAO (1999) Production yearbook. Food and agriculture organization of United Nations. Rome, pp: 74-92.
- Wilson RT (1991) Equines in Ethiopia, In: Fielding, Pearson, R.A. (editorial): Donkeys, Mules and Horses in tropical agricultural development. Edinburgh, Scotland, Center for Tropical Veterinary Medicine, University of Edinburgh pp: 33-47.
- Alemayehu L (2004) Case study on reproductive activity of equines in relation to environment factors in central Ethiopia, Berlin: hum bold university of Berlin, PhD thesis pp: 8-13.
- Central Stastical Authority (CSA) (2009) Federal Democratic Republic of Ethiopia, Central Stastical Authority (CSA), Agricultural Sample Survey 2008/2009). A Report on Livestock and Livestock characteristics (Private peasant holdings), Addis Ababa pp: 183.
- Feseha G, Mohammed A, Yilma J (1991) Vermicular endoparasitism in donkeys of Debre Zeit and Menagesha, Ethiopia. Donkeys, mules and horses in tropical agricultural development. Proc. Colloq. On donkeys, mules and horses. Eds. D. Fielding and R.A. Pearson, University of Edinburgh, Center for tropical veterinary medicine: UK pp: 156-160.
- Fred O, Pascal K (2006) Extension approaches to improving the welfare of working equines. Kenya Network for Dissemination of Agricultural Technologies (KENDAT), Nairobi, Kenya pp: 1-28.
- The Equinest (2012) Horse breeds from Ethiopia. Available online at: www.info[at]theequinest.com
- Arsi-Bale Zone Plan Office (2009) Zonal Agricultural Compiled Report, Oromia region, Ethiopia, pp: 7-11.
- Abayneh T, Gebreab F, Zekarias B, Tadesse G (2002) The potential role of Donkeys inland tillage in central Ethiopia. Bull Animal Health Prod Afr 50: 172-178.
- Sapakota CR (2009) A Report on Prevalence of Helminthes Parasites in Mules of Brick Kiln pp: 160.
- Bakirci S, Çirak VY, Gülegen E, Karabacak A (2004) Parasites found by fecal examinations in horses in the Gemlik Military Stud Farm. J Turkiye Parazitol Derg 28: 35-37.
- Feinstein RE (1979) Dictyocaulus arnfieldi in donkeys in Neuquen Province, Argentina. Revista Militar de Veterinaria 60: 125-128.
- Fikru R, Reta D, Teshale S, Bizunesh M (2005) Prevalence of equine gastrointestinal parasites in western highlands of Oromia, Ethiopia. Bull Animal Health Prodin Afr 53: 161-166.
- Gothe R, Heil HG (1984) Intestinal parasites and lungworms of donkeys in Germany: Age-related evaluation of prevalence of infections and specific composition. Deutsche Tierrztliche Wochenschrift 91: 144-145.
- Hansen J, Perry B (1996) The Epidemiology, Diagnosis and control of Helminthes parasites of ruminants, ILRAD, Nairobi, Kenya pp: 83.
- Smith PB (2009) Large animal internal medicine, 4th, USA, Mosby Elsevier of Lalitpur District pp: 232-321.
- Rose FR, Hodgson RDS (2000) Manual of Equine practice. 2nd, USA, Saunders pp: 224.
- George LW, Tanner ML, Roberson EL, Burke TM (1981) Chronic respiratory disease in a horse infected with Dictyocaulus arnfieldi. Am Vet Med Assoc 179: 820-822.
- Beelitz P, Gobel E, Gothe R (1996) Endoparasites of donkeys and horses kept together in Upper Bavaria. Range of species and intensity of infestation. J Tierarztliche Praxis 24: 471-475.
- Morris C, Trawford A, Svendsen E (2004) Donkey: Hero or villain of the parasite world past, present and future. Vet Parasitol l125: 43-58.
- Tesfaye A, Curran M (2005) A longitudinal survey of market donkeys in Ethiopia. Holetta Research Center, Addis Ababa, Ethiopia. Trop Animal Health Prod 1: 87-100.
- Johnson M, MacKintosh G, Labes E, Taylor J, Wharton A (2003) Dictyocaulus Cross infection between cattle and red deer New Zealand Vet J 51(2): 93-98.
- Bowman DD (2003) Georgis, Parasitology for Veterinarians, 8th, USA, New York Saunders pp: 166-168.
- Junquera P (2014) Dictyocaulus species, parasitic lungworms of Cattle, Sheep, Goats and other Livestock. Biology, prevention and control. Dictyocaulus filaria, Dictyocaulus viviparus, Dictyocaulus arnfieldi. In: Merck Veterinary Manual, Merck and Co, Inc, Whitehouse Station, N.J., USA, Last Updated on Friday, January 03: 14:28.
- Fraser CM (2000) The Merck Veterinary Manual. A hand book of Diagnosis Therapy and Disease prevention and control for the Veterinarians 8th, Merck and Co; Inc, Rahaway, NIT, USA pp: 851-852.
- Blood DC, Radostits OM (1999) Veterinary Medicine, A text book of the disease of Cattle, Sheep, Pigs, Goats and Horses, 7th , Bailliere Tindal, London, UK pp: 1064-1066.
- Blood DC, Radiostitis OM, Gray, Hincheeliff KW (2000) Veterinary Medicine a text of book of the disease of Cattle, Sheep, Pigs and Horses. 8th, Harcourt Publisher Ltd, London pp: 1246-1253.
- Urquhart GM, Armur JI, Dunn AM, Jenninngs FW (1999) Veterinary Parasitology 2nd Ed, pp: 39-40.
- Blood DC, Rodastitis OM, Gay CC (1989) Veterinary Medicine a text book of the disease of cattle, sheep, pigs, goats and horses 6th, Balilliere Tindal pp: 1039-1044.
- Howard J (1993) Current Veterinary therapy of food animal practice, 3rd, W.B. Sunders Company Harcourt Brace Ivovanov pp: 673-675.
- Aiello S, Mays E (1998) The Merck Veterinary manual, 8th Ed., (Merck and Co Inc., White house station, NJ) pp: 131-140.
- Stuart MT (2012) An overview of Lungworm Infection. In: Merck veterinary manual, Merck and Co; Inc, Whitehouse Station, N.J., USA, Last full review/revision on March.
- Boon J, Kloosterman N, Vanderlander T (1984) The incidence of viviparous infection of cattle, Netherland, Surveys of sera collected in the field. Vet Quarter pp: 13-17.
- Reinecke RK (1989) viviparous, Veterinary Helminthology, professional publisher (Pty) Ltd, Pretoria pp: 178-180.
- Burks BS (1998) Parasitic pneumonitis in horses. Compend Contin Educ Vet 20: 378-383.
- Belay M (2005) Preliminary study on helminthiasis of equines in South and North Wollo zones. Ethiop Vet J 9(2): 39-50.
- Power DG (1992) The health of horse, Great Britain pp: 67-82.
- Herd RP (1987) Epidemiology and control of equine strongylosis at new market. Equine Vet J 18: 447-452.
- Mersha C, Tihitna S, Basaznew B, Achenef M (2012) Occurrence of Lungworm Infection in Equines and their Associated Risk Factors. Global Veterinaria 8(1): 35-38.
- Yitna T (2015) Prevalence and Associated Risk Factors of Equine Lungworm ( Arnfieldi) In Lode Hetosa District, South Eastern Ethiopia. Acad J Animal Dis 4(2): 104-111.
- Tilahun B, Nuraddis I, Benti D, Tadele T (2014) Prevalence of Helminth Parasites of Horses in and Around Hawassa Town, Southern Ethiopia. Acta Parasitologica Globalis 5(1): 7-11.
- Abdulkadir A, Nuraddis I, Yosef D (2016) Prevalence of equine lungworm and associated risk factors in Sudie district, Oromia region, southeastern Ethiopia. Afr J Agric Res 12(18): 1526-1531.
- Thrusfield M (2005) Veterinary epidemiology 3nd, Veterinary Clinical Studies Royal (Dick) School of Veterinary Studies University of Edinburgh pp: 233.
- Anne M, Gray A (2006) Veterinary clinical parasitology. 7th, Australia, Blackwell Publishing pp: 11-14.
- Charles MH, Robinson E (2006) Diagnostic veterinary parasitology for veterinary technicians, 3rd, Mosby Inc. St. Louis, Missouri pp: 243.
- Crane M (1997) Medical,In: E.D. Svendsen, (Ed.). The professional Hand Books of the Donkey. 3rd, Whittet Books Limited, London pp: 29-31.
- Svendsen ED (1997) Parasites abroad: The professional hand book of the donkey, 3rd Ed, pp: 166-182.
- Getahun F (1993) Helminthes and external parasites of equines species in Bale Administrative region, Ethiopia pp: 55.
- Shiferaw Y (1993) Preliminary survey on helminthiasis and management practice of equine in Wenchi Awraga, Ethiopia pp: 32-78.
- Adere F, Tilahun B (20016) Prevalence of Equine Lung Worm (Dictyocaulus Arnfieldi) and Its Associated Risk Factors in Jimma Town, South West Ethiopia. J Adv Life Sci Technol 40: 16-26.
- Legese Y (1996) Preliminary survey on management aspects and health problem of donkey in Dire Dawa and East Oromia, Ethiopia pp: 34.
- Hassan T, Salih MM, Abakar AD (2004) A Survey of Gastrointestinal Nematodes of Donkeys (Equus asinus) in Khartoum State, Sudan. J Animal Vet Adv 3: 736-739.
- Andersen S, Fogh J (2010) Prevalence of lungworm ( arnfieldi) in donkeys in Denmark and in horses in herds together with donkeys. J Nord Vet Med 33: 484-491.
- Ayaz E (2003) The prevalence of Dictyocaulus arnfieldi in horses and donkeys. J Turkiye Parazitol Derg 14: 77-81.
- Esheta G (2000) Preliminary survey on the health and reproductive status of “Gari” Mules in and around Bahir Dar pp: 45.
- Lyons ET, Tolliver SC, Drudge JH, Swerszek TW, Crowe MW (1985) Lungworms (Dictyocaulus arnfieldi). Prevalence in live equids in Kentucky. Am J Vet Res 46: 921-923.
- Yacob HT, Hagos A (2013) Epidemiological study on Gastrointestinal Helminthes of horses in Arsi-Bale highlands of Oromia Region, Ethiopia. Ethiop Vet J 17(2): 51-62.
- Tihitna S, Basaznew B, Mersha C, Achenef M (2012) Occurrence of Lungworm Infection in Equines and their Associated Risk Factors. Global Veterinaria 8(1): 35-38.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Agriculture and Forest Meteorology Research (ISSN:2642-0449)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Journal of Womens Health and Safety Research (ISSN:2577-1388)
- Food and Nutrition-Current Research (ISSN:2638-1095)





