INTRODUCTION
To address the growing challenge of demand for protein in both human food and livestock feed, players in the pig industry are currently seeking new alternatives that are ecological, functional and economically viable. In parallel, in both human and animal health, the problematic development of bacterial resistance has forced world health organizations, such as the OIE (World Organization for Animal Health) to adopt measures to promote the responsible use of antibiotics in animal farming.
At a European level, the use of antibiotics at “growth promoter” doses have been banned since 2012 and the use of zinc oxide for therapeutic use will also be banned by 2022. In France, the various strategies already implemented (Eco-antibio plans I and II) have delivered highly satisfactory results thanks to all the support work and the monitoring of farmers by all the professionals in the field. Nonetheless, despite all the efforts already made and the results obtained, these new constraints are leading to the re-emergence of nutritional issues that were partially controlled by the antibiotic treatments. Similarly, environmental constraints are now imposing new standards on the use of copper, renowned for its effects on the growth of piglets.
The use of hyper prolific sow lines is giving rise to the production of piglets that are smaller at the weaning stage, and therefore frailer. Yet there is no question that weaning is one of the most critical periods for young pigs. The stress they face causes physiological changes whose consequences can be considerable [1,2]: digestive disorders of varying degrees of intensity and duration, reduction in feed intake lasting 2 to 3 days [3,4], alterations to the intestinal mucosa [5], slowdown in intestinal transit and gastric sluggishness [6]. The gut flora is destabilized, allowing pathogenic agents to establish themselves, multiply and cross through the mucosa and then the digestive lining. Thus, besides establishing a stable and varied microbiota with the inclusion of probiotic yeasts, for example [7], one of the major challenges at the weaning stage is to promote feed intake as quickly and effectively as possible. Supplementing with solid feed from the youngest age possible is one of the primary levers, another is to formulate feeds for piglets with high-quality raw materials that are highly digestible, free of anti-nutrient factors and antibiotics and, lastly, palatable. As such, protein- rich feeds play an important role as a source of amino acids (AAs). Since piglets have an immature gastro-intestinal tract, the apparent ileal digestibility (AID) of raw protein for a given feed is lower at this stage than that of grower pigs. The proteins of AAs that are not digested at the end of the ileum reach the large intestine and are fermented. They cannot then be used for protein production or as a source of energy by the pig. Furthermore, the non-digestible nitrogen fraction can be used as a substrate for bacterial fermentation such as E. coli or other pathogenic agents, which increases the risk of diarrhea at the post-weaning stage and can have a negative effect on growth performance [8]. Consequently, knowing the digestibility coefficient (DC) and the standardized ileal digestibility (SID) of AAs is important in order to evaluate the efficacy of AA digestion and as a potential indicator of the gut health of the piglets. Knowing the energy value of the ingredient is important too so it can be incorporated into the formula.
The use of functional proteins in post-weaning diets is showing promising results, particularly in terms of protein intake from selected yeast extracts. Thus, in order to validate the efficacy of yeast extract supplements in the feed of weanling pigs, three Studies were performed, one to measure the concentrations of digestible energy, metabolic energy, net energy and the standardized ileal digestibility of 18 AAs from an extract of protein-rich (63% of fresh weight) bakery yeast L, compared with a brewer’s yeast S (protein concentration: 44% of fresh weight). The two other Studies evaluated and validated the dose to optimize post-weaning growth performance.
MATERIALS AND METHODS
Three Studies were performed to evaluate the digestibility (Study 1), the effective dose (Study 2) and the effects on the growth performance of weaned piglets (Study 3) of a protein- rich bakery yeast (Prosaf®, Phileo by Lesaffre, France).
EXPERIMENTAL SYSTEM AND EXPERIMENTAL FEEDS
Digestibility Study (Study 1)
Study 1 was performed at the Schothorst Feed Research experimental station (SFR, the Netherlands). In order to define the digestibility coefficients of proteins, energy and that of the AAs of the bakery yeast extract L, 18 Topigs L20 x Tempo cross piglets were placed individually in metabolic pens on weaning (26 days old, average weight of 7.9 kilograms - kg).
A prestarter reference formula used as the control (C) included 20% of the extract L (gross energy: 21.6 MJ/kg) or 25% of a standard brewer's yeast S (gross energy: 18.4 MJ/kg). The protein content of the feeds (C), (L) and (S) was respectively 13.1, 23.3 and 19.2% of the fresh weight. These three feeds (C, L and S) contained 0.5% titanium dioxide as an inert marker. After 4 days of suckling, each of the three experimental feeds was fed in pellet form to six piglets for 10 days (feed adaptation period). All the piglets’ feces and urine were then individually collected, twice a day for the following 9 days, in order to determine the apparent digestibility coefficients of dry matter and proteins, and to calculate the net energy [9] of the two yeast products. On the last day of the Study, the piglets were slaughtered and the content of their ileum was sampled to determine the AA SID of the two tested products, using, for each AA, the basal endogenous losses indicated by the Dutch CVB tables (2016).
Determination of the Effective Dose (Study 2)
Study 2 was conducted at the Akei Animal Research experimental station (Brazil). One hundred and ninety-two (192) weaned Camborough x Ag 337 cross piglets aged 23 days and with an average weight of 6.1 kg, were individually secured in 32 fully slatted pens (2.55 m²/pig) of six piglets.
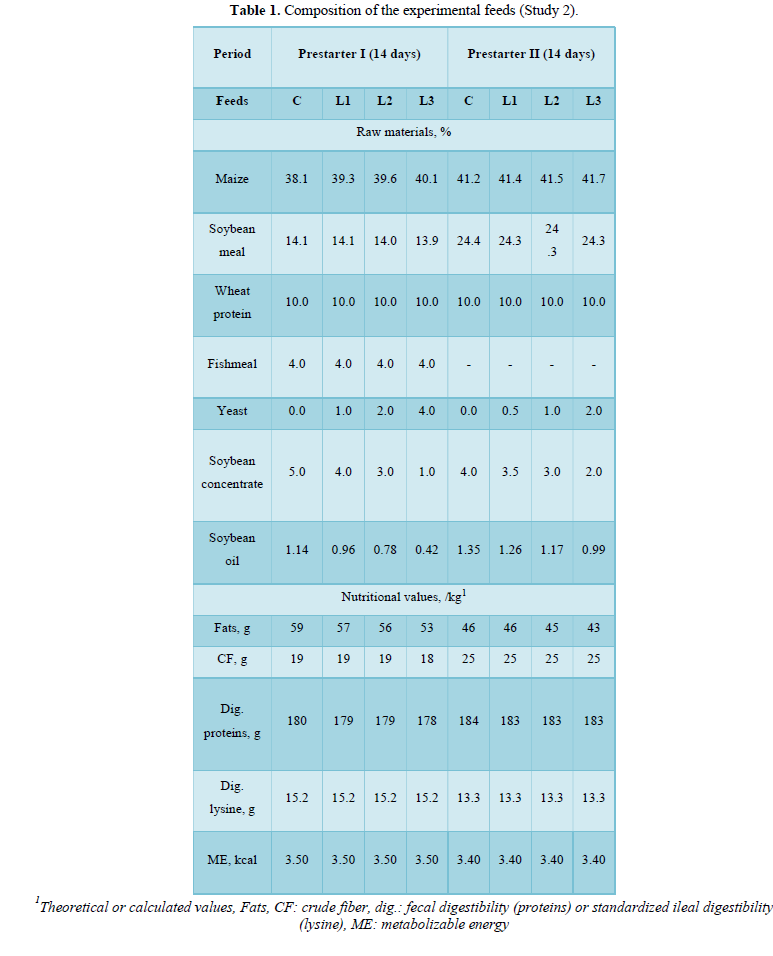
Feed was given ad libitum in a 100 cm manger and the animals had access to water pipettes. The temperature and humidity were recorded daily. Each group (n = 8 repetitions) was fed one of the following combinations of prestarter feed I (14 days) then prestarter feed II (following 14 days) supplemented with yeast extract L: 0%-0% (C), 1%-0.5% (L1), 2%-1% (L2), 4%-2% (L3).
The formulas were isocaloric (theoretical metabolizable energy: 3.50 Mcal/kg), isoproteinic (20.0% of fresh weight), and formulated according to the nutritional recommendations commonly used in Brazil (Table 1).
At the end of these two supplementation periods (prestarter I and II), all the piglets received a standard starter feed, without the addition of yeast extract, until the end of the post-weaning period (14 days). The growth performance of the piglets such as the average daily gain (ADG), the feed intake and the feed conversion ratio (FCR) were measured or calculated for each of the periods.
Study in Livestock Production (Study 3)
The objective of Study 3 was to test a prestarter feed (fed for 14 days), formulated without animal proteins, that was isoproteinic and at a cost comparable with a formula containing blood plasma.
In a Morbihan “farrow to finish” production facility (170 Adenia genetic sows, inseminated by Piétrain Axiom boars), 987 piglets (three consecutive bands) aged 21 days and with an average weight of 5.9 kg, were divided into two homogeneous groups, one group being fed a prestarter feed containing the yeast (L) (n = 484) or the plasma (P) (n = 503). The animals of group (L) received a prestarter feed containing 1.5% yeast extract L, while the piglets of group (P) were given a prestarter feed that did not contain the yeast extract L but did contain 2% blood plasma. After 15 days of supplementation, the animals of the two groups received the same starter feed without the addition of the yeast extract or plasma.
Weighings by pen were performed on weaning, at 37 and at 65 days old. The feed intake was recorded during the prestarter- starter feed transition and at the end of the Study period. The feed conversion ratio (FCR) and the average daily gain (ADG) were calculated.
Statistical Analyses
The statistical analyses were carried out using the R Studio program, version 3.5.0 (2018-04-23). In Study 1, the digestibility data were compared using the Student test, where the animal is considered the experimental unit.
In Studies 2 and 3, the effect of the feed was evaluated by an ANOVA. A comparison of the averages of each group was then carried out by the Tukey test, where the pen is considered the experimental unit. All the analyses were carried out with a 5% confidence interval (p≤0.05).
RESULTS
Analytical Composition of the Yeast Extract and the Brewer's Yeast and Piglet Digestibility Coefficients (Study 1)
Study 1 demonstrated a better fecal digestibility (p = 0.005) of the protein content of the yeast extract L (90.1%) compared with the brewer’s yeast S (77.5%, Table 2).
The yeast extract L presented a better fecal digestibility of the dry matter content (p = 0.014). The net energy concentration measured was similar for the two yeasts (Table 2).
In terms of the essential AA SID coefficients in the piglet, those of the yeast extract L varied between 68.5 and 87.2% while those of the yeast S varied between 23.5 and 55.9%. Considered individually, the essential AA SID coefficients were clearly higher for the yeast extract L compared with the yeast S (p <0.05), with the exception of histidine, whose digestibility coefficient was similar for the two yeasts studied.
Determination of the Effective Dose (Study 2)
While the experimental feeds were only fed to the animals for 28 days, the performance of the piglets was monitored throughout the post-weaning period (42 days). Thus, by considering this whole growth phase (D1- D43), the combination LD delivered a significantly improved ADG (416 vs 371, 385 and 394 g/d, respectively, for C, L1 and L3, P = 0.03), while the feed intake variances (846 vs 788, 818 and 830 g/d, P = 0.43), the FI (1.91 vs 1.99, 1.96 and 1.98, respectively, P = 0,69), comparative to the control group and the other yeast groups, are not significant.
By analyzing each period separately, the differences between the groups were most marked during the prestarter I phase (D1-D14), notably with a significantly improved ADG (P = 0.023) and a better FCR (P < 0.001) for the piglets fed the L2 combination compared with the other groups (Figure 1). During the prestarter I phase (D1-D14), as well as the prestarter II phase (D15-D28), the feed intake differences between the groups were not statistically significant.
Conversely, the ADG tended to be higher (p = 0.07) and the FCR was statistically lower (p = 0.03) for the group L2 compared with the other groups (Figure 1).
At the end of the Study period, the average weight of the piglets revealed a significant difference for the combinations L2 and L3 (23.42 kg and 22.57 kg, respectively) compared with the control batch and the combination L1 (21.85 kg vs 22.45 kg respectively, P = 0.05).
Growth Performance (Study 3)
Study 3, conducted in a commercial breeding unit, validated the interest in adding the yeast extract L to a prestarter formula, incorporated at a dose close to that identified in Study 2, the results being similar to a formula with blood plasma.
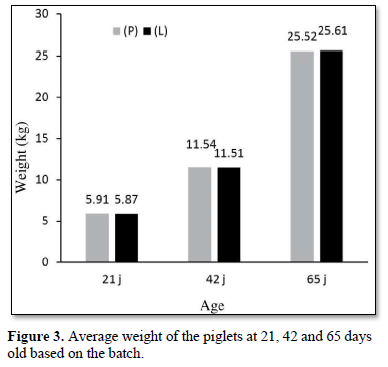
No significant difference was observed between the group (L) and the group (P) in terms of the average weight at 42 days old (P = 0.88, standard deviation: ± 1.3) and at 65 days old, corresponding to the end of the Study (P = 0.82, standard deviation: ± 3.3) (Figure 3).
The growth results obtained were also similar for the ADG of the batches (P) and (L) during the 21- and 42-days old period (268 for the group (P) vs 269 g for the group (L), P = 0.76) and during the 21- and 65-days old period for the ADG (446 for the group (P)s 449 g for the groups (L), P = 0.78) and during the 21- and 42-days old period for the FCR (1.16 for the group (P) vs 1.19 for the group (L), P = 0.76).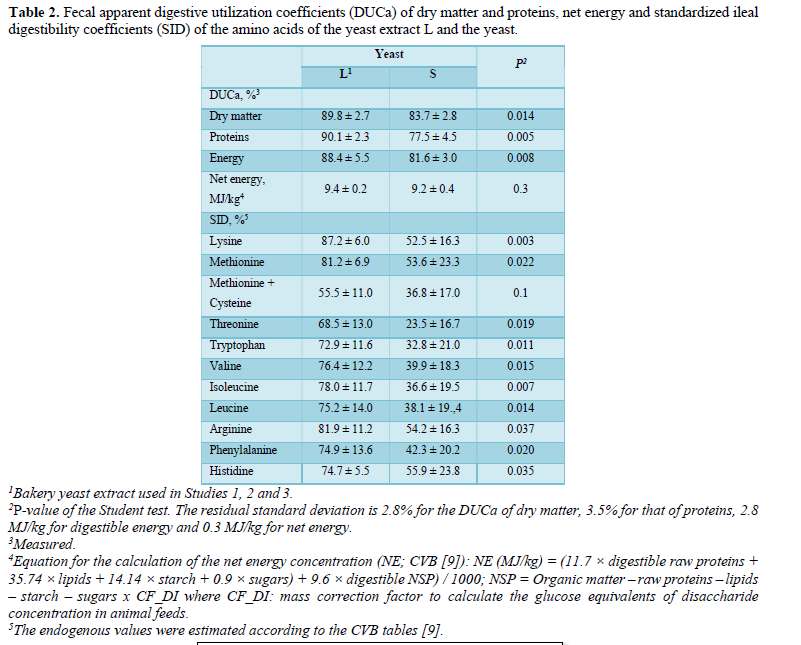
DISCUSSION
A purified source of functional proteins must first and foremost be palatable with a high level of digestibility. It must also promote the rapid development of the digestive tract in the young, and strengthen their immune status. In these Studies, the yeast extract used had a minimum protein concentration of 63% of the fresh weight, a high level of amino acid digestibility and high rates of total nucleotides (7.7%) and glutamic acid (10.9%). The Study established a better digestibility of the amino acids contained in the yeast extract L. Several authors [8] have shown that the absorption of dipeptides and tripeptides was faster than that of their constituent AAs. Thus, the efficacy of the yeast extract L is explained first and foremost by its peptide composition, with 26% of free AAs and 38% of AAs of low molecular weight (< 0.9 kDa): the AAs can be transported through the brush border membrane of the intestinal epithelial cells, either in free form or in the form of dipeptides and tripeptides.
The high glutamic acid concentration (10.9%) of the yeast extract L also seems to play an important role, not just in terms of palatability and the taste acceptance of the prestarter I and II feeds, but also in gut development and, consequently, its performance [10,11]. In fact, it has been showed that the glutamic acid had palatability benefits [12]. The results obtained with the yeast extract L can therefore be explained in part by its high glutamic acid content. The addition of the extract L might also provide benefits similar to those of sow milk. Indeed, among the 20 free AAs contained in sow milk, glutamic acid is the most abundant, representing more than 50% of the total free AA concentration. Glutamine is an essential nutrient for the gut; around 30% of the total glutamine is used in the different intestinal tissues. It is linked to the promotion of the proliferation of enterocytes, the regulation of the tight junctions, which are the proteins that regulate the permeability of the intestinal epithelium, and is involved in the suppression of pro- inflammatory signaling pathways to protect the cells from apoptosis and cellular stress in normal and pathological conditions [13,14]. The results of several studies in animals suggest that dietary nucleotides might affect the gastro-intestinal tract by promoting the faster maturing of the tract, by modulating the intestinal microbiota and by activating the immunity strengthening cells. Although nucleotides are synthesized endogenously, it has been suggested that a nucleotide feed supplement could have a beneficial impact on the growth and development of the small intestine, lipid metabolism and hepatic function [15,16]. Thus, even if the nucleotides present in the yeast extract are present in the form of RNA, their presence might explain in part the positive effect on growth and the intake efficiency of the piglets.
CONCLUSION
Generally speaking, the yeast extract L used in the combination L2 produced better results than the other L groups. It is therefore when it is incorporated at 2% in the prestarter I feed used for the first 14 days and at 1% in the prestarter II feed used for the next 14 days that it is most effective, noting all the same that the diet inclusion programme will depend on the farm's nutritional programme, and that the optimum benefit of the yeast extract L supplement is obtained when the piglets are capable of eating between160 g and 200 g of the product over the first 28 days of the post-weaning phase.
The incorporation of the yeast extract L might enable nutritionists to formulate a non-animal protein feed as effective as those formulated with plasma proteins. Thus, thanks to the combination of its functional active ingredients, together with good palatability, the amino acid profile and a high protein and AA digestibility, the yeast extract L appears to represent a novel raw material for use in weanling pig feed.