Case Report
Review on Bovine Brucellosis and its Current Status in Ethiopia
15717
Views & Citations14717
Likes & Shares
Bovine Brucellosis is one of the most common bacterial zoonosis worldwide and it poses a major threat to human health, animal health, and animal production. Clinical signs are similar for all species and commonly include abortion, stillbirth or weak calves, retained placentas, and decreased milk yield. The primary rationale for brucellosis eradication is driven by the economic benefits to the cattle industry and consumers of its products. Diseases are emergency animal diseases that have the potential to cause major national socioeconomic consequences through very serious international trade losses, national market disruptions and very severe production losses in the livestock industries that are involved. Public health significance includes illness, physical incapacity and loss of manpower and also results in the scarcity of animal proteins due to loss of meat. Occupational risk of brucellosis is important because of the high possibility of direct transmission from infected animals to the people employed in animal husbandry. This exposed group includes slaughter men, dairy men, herd’s men and veterinary y clinicians. Herd’s men are the most exposed members. This occupational exposure is high in Ethiopia, where herding of animals is traditional and unscientific. Public awareness is of vital importance in successful control and prevention of brucellosis; Isolation of infected animals and female at parturition; proper disposal of aborted fetus, placental tissue and uterine discharge; disinfecting of contaminated areas and pasteurization of milk.
Keywords: Bovine Brucellosis, Brucella abortus, Status in Ethiopia
INTRODUCTION
Brucellosis is one of the major zoonotic infections worldwide. It is caused by gram-negative cocco-bacilli of the genus Brucella. Brucellosis in cattle is primarily caused by the bacterium Brucella abortus, which is one of six species of the genus Brucella. Nine biotypes have been identified, all of which are intracellular, parasitizing, gram-negative, short rods [1].
Although brucellosis has been controlled in most industrialized countries, it remains a major problem in the Mediterranean region, western Asia, Africa, and Latin America. It can cause appreciable economical losses in the livestock industry because of abortions, decreased milk production, sterility, and veterinary care and treatment costs [2].
The proportion of people relying on livestock for some or their entire livelihood is very high in Africa, ranging from 20 to over 90%, depending on the livestock production system and country in focus. Ethiopia hosts large number of cattle that are raised under extensive pastoral production system or in adjunct to crop production. Ethiopian cattle population is estimated about 40.6 million and ranked first in Africa [3]. The number of surveys done in Ethiopia on the prevalence of bovine brucellosis in intensive and extensive livestock management has already indicated how important the disease is in different parts of the country [4].
In Ethiopia, there is no documented information how and when brucellosis introduced and established. Several serological surveys have showed bovine brucellosis is endemic and widespread disease in urban, peri-urban, highland and lowland, extensive and intensive farming, small holder farms and ranches of the country [5,1].
The available information on bovine brucellosis in the countryclearlyshowedthatthe disease iswidelyspreaded withsignificanteconomic andpublic health importance [6]. Therefore, this review is undertaken with the following objective.
To Review bovine brucellosis and its current status in Ethiopia.
LITERATURE REVIEW
Definition
Brucellosis is an infectious, contagious, and worldwide spread form of an important zoonosis disease caused by bacteria of the genus Brucella. In animals, the disease primarily affects cattle, sheep, goats, swine, and dogs, and is characterized by abortion or infertility and also affects people and other animal species [7]. In human beings, the disease is characterized by intermittent fever, chills, sweating, headache, myalgia, arthralgia, and a diversity of nonspecific symptoms [8].
Aetiology
Brucella are small, cocco-bacillary or short rods; with a size range of 0.5 to 0.7µm. by 0.6 to 1.5µm. These organisms are gram-negative and frequently take the counter stain poorly. They are aerobic, non-motile, and non-fermenting. They occur singly or in groups, are non-sporulating, and non-encapsulated [9].
Brucellosis infection is caused by species of the bacterial genus Brucella. There are six different species of Brucella, whereby Brucella abortusis the predominant species infecting cattle and cause Bang’ s disease in humans. Apart from cattle, other animal like goats, sheep, pigs, buffaloes, camels and reindeer can be affected by brucellosis [10].
In other species B. melitensis is the causative agent of brucellosis in small ruminants and Undulating or Malta fever in humans. B.ovis, causes brucellosis in sheep. B. suis is the causative agent of brucellosis in pigs which also can be transmitted to humans.B. caniscause brucellosis in dogs andB. neotomae occurs in desert rats in the USA [11]. Nine biotypes have been recognized, as well as a number of strain variants. About 85% of infections are from biotype 1, whereas biotypes 1, 2, 3, 4, 6, 7, 8, and 9, are recognized in Africa. All of which are intracellular, parasitizing, gram-negative, short rods. Brucella has a wide host range, but cattle are the preferred host of B. abortus. Brucella can infect humans and cause significant disease (‘undulant fever’). The most important brucellosis disease in humans is ovine/caprine brucellosis caused by B. melitensis [12].
Epidemiology
Geographic Distribution and Occurrence of Bovine Brucellosis:Geographically bovine brucellosis has been reported in Asia, Africa, South and Central America, the Mediterranean Basin, Sahara and the Caribbean [13] and these are the regions where cattle raising are mostly preferred. Infected or exposed animals have also been found along the Atlantic and Pacific coasts of North America; the coasts of Peru, Australia, and New Zealand [14]. Incidence of brucellosis is reported to be the highest in bovines and prevalence range of 0.85-23.3% has been reported from a wide range of countries. Bovine Brucellosis is widespread in African countries, although with varying prevalence [15].
The disease has been eradicated in some industrial countries, especially in Europe, through intensive schemes of control and eradication.However, its occurrence is increasing in developing countries in an even aggravating epizootiological situation, which depends on the policy of many developing countries of importing exotic high production breeds without having the required veterinary infrastructure and the appropriate level of development of the socioeconomic situation of the animal holder [2]. Furthermore, the increasing international animal trade with increasing movements of animals and the trend towards intensification of animal production favours the spread and transmission of the infection [16].
Risk factors: Risk factors associated with bovine brucellosis have been described to include: -host, agent, management and survival of Brucella in the environment [17]. The prevalence of those risk factors for infections is best understood for bovine brucellosis and to a lesser extent for ovine and caprine brucellosis [13].
Host risk factors:Susceptibility of cattle to Brucella abortus infection is influenced by the age, sex and reproductive status of the individual animal.
Sexually mature, pregnant cattle are more susceptible to infection with the organism than sexually immature cattle of either sex [4].
Youngsexually immature cattle generally do not become infected following exposureor recover quickly [18]. Sexually mature females are more susceptible to B. abortus infection than bulls [5]. This susceptibility increasesduring pregnancy, and animals get more susceptible with theadvance of pregnancy [19].
Agent Risk Factors:Brucella abortus is a facultative intracellular parasite, which is capable of multiplication and survival within hostphagocytes. The organisms are phagocytosed by polymorph nuclear leukocytes, in which some survive and multiply. These are then, transported to lymphoid tissues and foetal placenta 18]. The inability of the leukocytes to effectively kill virulent B. abortus at the primary site of infection is a key factor in the dissemination to regional lymph nodes, other sites such as the reticulo-endothelial system, and organs such as the uterus and udder [20].
Management Risk Factors
The spread of the disease from one herd to another and from one area to another has been linked to almost always due to the movement of infected animals. The unregulated movement of battlefrominfectedherdsorarea tobovinebrucellosis-freeherds or areasis themajor cause of breakdowns in bovine brucellosis eradicationprograms. Other management factors influencing inter-herd transmission are proximity to infected herds, water ways, and scavengers. A variety of cattle husbandry practices also have been shown to be associated with the spread of B. abortus infection within herds. Vaccination level, population density, methods of housing, and use of maternity pens influence the probability of exposure to infection [21]. Many factors affect the epidemiology of bovine brucellosis; the most important are herd size and mobility, contiguity to infected herds, concentration of cattle and nature of production (dairy herds are more susceptible than beef cattle) [22].
ENVIRONMENT RISK FACTORS
In countries with temperate or cold climates there is a marked seasonal variation in the incidence of bovine brucellosis, with most cases occurring in the spring and summer. The ability of Brucella to persist outside the mammalian hosts is relatively high compared with most other non-spore forming pathogenic bacteria, under suitable condition. Numerous studies have assessed the persistence of Brucella under various environmental conditions. Thus, when pH, temperature and light conditions are favourable. i.e. pH>4,high humidity, low temperature and absence of direct sun light Brucella may retain infectivity for several months in water, aborted foetuses and foetal membrane, faeces and liquid manure, wool, hay, on building, equipment and cloths.Brucella is able to withstand drying particularly in the presence of extraneous organic material and will remain viable in dust and soil. Survival is prolonged at low temperature, especially below 0 C [22].
The survival of the organism in the environment may play a role in the epidemiology of the disease. A contaminated environment or equipment used for milking or artificial insemination is further source of infection. Permanent calving camps and lush pastures, particularly if they are wet and muddy, may play a very important role in the spread of the disease. Brucella has been found to be sensitive to direct sunlight, disinfectant and pasteurization. In any conditions they survive only if embedded in protein [21].
Sources of Infection and Mode of Transmission
The most significant feature of bovine brucellosis epidemiology is the shedding of large numbers of organisms during the 10 days after abortion or calving of infected cows and the consequent contamination of the environment. The movement of infected cattle into a herd can result in transfer of the disease when cattle ingest the bacteria from aborted foetuses, placenta, and discharge from cows that have aborted or contaminated pasture or water [23].
The organisms are probably most frequently acquired by ingestion; conjunctival inoculation, skin contamination and udder inoculation from infected milking cups are other possibilities. The use of pooled colostrum’s for feeding new-born calves may also transmit infection. Most or all Brucellaspecies are also found in semen. Males can shed these organisms for long periods or lifelong. The importance of venereal transmission varies with the species. It is the primary route of transmission for B. ovis. B. suis and B. canis are also spread frequently by this route. B. abortus and B. melitensis can be found in semen, but venereal transmission of these organisms is uncommon. Some Brucella species have also been detected in other secretions and excretions including urine, feces, hygroma fluids, saliva, and nasal and ocular secretions [24]. Artificial insemination can transmit the disease and semen must only be collected from animals known to be free of infection [3].
Humans usually become infected by ingesting organisms or by the contamination of mucous membranes and abraded skin. In the laboratory and probably n abattoirs, Brucella can be transmitted in aerosols [25]. Common sources of infection for people include contact with animal abortion products; ingestion of unpasteurized dairy products from cows, small ruminants or camels; ingestion of undercooked meat, bone marrow or other uncooked meat products; contact with laboratory cultures and tissue samples; and accidental injection of live brucellosis vaccines [20].
Human to human transmission is rare, but has been reported after blood transfusion, bone marrow transplantation or sexual intercourse [24].Rare congenital infections seem to result from trans-placental transmission or the ingestion of breast milk. Congenital infections might also occur if the infant is exposed to organisms in the mother’s blood, urine or feces during deliver y [26].
Pathogenesis
In cattle, infection with B. abortus is usually due to ingestion of infected material. The bacteria penetrate the mucosal epithelium of the gastrointestinal tract and are transported, either free or within phagocytic cells, to regional lymph nodes. If these bacteria do not remain localised or are not killed, they can spread to other organs, joints and bursa. This bacteraemic phase is subclinical and may take several weeks to some months. The bacteria then localise in the pregnant uterus and udder of cows, and the testicles and accessory sex glands of bulls [14].
In pregnant cows, the chorio-allantoic membrane becomes inflamed and ulcerated, and bacteria can spread via the blood to the foetus and placenta. The preference of the bacteria for these sites is thought to be due to the presence of the sugar alcohol erythritol, which is a foetal product concentrated in the chorion, cotyledons and foetal fluids [27].
In mature, non-pregnant cows, the bacterium localises in the udder. Infection of the udder is often clinically inapparent, with no gross lesions. Brucella localises and replicate primarily in macrophages in mammary secretions or in phagocytes; they form an important source of organisms for periodic reinfection (and potentially for infection of calves and humans via the milk). Hence, if the cow later becomes pregnant, the uterus can become infected during a subsequent bacteraemic phase [23].
Clinical Finding
The variable facets of clinical symptoms which are typical for brucellosis are the consequence of the individual level of host defence which is specific for each breed, and also influenced by genetically determined resistance, level of immunity, age of the animal,productivity, condition, environmental influences as well as virulence of the pathogen [11].
The primary clinical sign in female cattle are a significant number of late term (5–7 months) abortions, stillborn or weak calves, retainedplacentas, and decreased milk yield. In a population that has not been exposed to the disease before, these may appear as an ‘abortion storm’, with many cows abor ting over a short period. Geering and his collagens (1995) reported 30–80%abortions in fully susceptible herds. Many cases of endometritis and retained placenta also occur. However, such overt clinical evidence may not be seen in dry areas (where conditions are unfavourable for survival on pasture) or in large, extensively managed herds. In bulls, clinical signs include inflammation of the testis (orchitis), ampulla’s, testicles, and epididymis have been reported to be infected and testicular abscesses may occur, as long-standing infections may result in arthritic joints and hygroma in some cattleand lameness due to bursitis, which is typically seen in infected bulls and occasionally in cows. Sexually immature cattle do not usually show any signs but may remain subclinical infected until maturity and pregnancy [17].
The length of the incubation period in an individual animal is influenced by sexual maturity, state of pregnancy at the time of infection, size of the challenge dose and previous exposure to infection or vaccination. For example, the average incubation period is 67 days for cows infected at six months of pregnancy. The minimum incubation period is about one month [28].
Diagnosis
Brucella species can be recovered from numerous tissues and secretions, particularly fetal membranes, vaginal secretions, milk (or udder secretions in nonlactating cows), semen, arthritis or hygroma fluids, and the stomach contents, spleen and lung from aborted fetuses. Thediagnosisofbovinebrucellosisisconfirmedbyisolationandidentificationofthe causative organism. In order to be able to screen a large number of animals, the diagnostic tests should be ‘inexpensive, easy to perform, rapid, highly sensitive and fairly specific’. Several serological tests have been designed to meet these requirements [29].
[30] Recently produced a comprehensive review of the serological tests for brucellosis that are in common use. Therefore, the most commonly used serological tests are only briefly summarised. Tests that are comparable (similar specificity and sensitivity as well as similar other characteristics) are grouped together. These tests are: Acidified antigen agglutination tests such as the rose-bengal plate test (RBPT) and the buffered antigen plate agglutination test. These serological tests are simple to perform, inexpensive and suitable for screening individual animals. However, false negative reactions occur; Standard agglutination tests(SAT) such as the standard tube agglutination test and the sero-agglutination test of Wright constitute anothergroupof tests thatarecomparable with each other, SAT testsare susceptible to producing false positive reactions [25].
The Complement fixation test (CFT) is another, separate test. The CFT is recommended by the OIE as the test prescribed for international trade, CFT is often used as a second test for confirmation of RBPT-positive sera; indirect enzyme-linked immuno sorbent assays (ELISA) are the fourth serological test group that is often used to determine the prevalence of brucellosis in surveys. Recently developed ELISA tests are highly sensitive, simple to use but expensive; Milk ring test (MRT) is an adaptation of the agglutination test. This test is used to show if antibodies are present in the milk [31].
Treatments
Treatment is unsuccessful because of the intracellular sequestration of the organisms in lymph nodes, the mammary gland, and reproductive organs. Brucella species are facultative intracellular bacteria that can survive and multiply within the cells of the macrophage system. Treatment failures are considered to be due to the inability of the drug to penetrate the cell membrane barrier. However, humans are usually treated with the following antibiotics: Doxicycline with rifampicine. Relatively short courses (less than 8 weeks) of treatment with antibiotic combinations have been associated with high rates of relapse [32].
Control and Prevention
Given the complexity of the epidemiology of brucellosis involving various animal species, the effective control will require a long lasting and carefully controlled and monitored effort. Health education of risk groups through community participation and health education programmes could play an important role to increase the acceptance and use of preventive measures [33].
Where there is any suspicion of bovine brucellosis, quarantine must be imposed immediately to ensure that any infection is contained. Quarantine may be partial or total, depending on the extent of infection and herd management. For example, on very large properties or where there are valuable stud animals involved, a group of infected or suspicious animals that are isolated from the rest of a herd may be quarantined and managed separately. However, all animals in such herds will require repeated sero-surveillance to confirm their freedom from infection. Prompt examination of movement records will assist in tracing any movements that may be suspect. Movement of latently infected cows and heifers presents the greatest risk, but the potential for movement of infected material by dogs or birds cannot be ignored. Strict sanitation measures must be applied immediately to isolate animals likely to calve, the area surrounding an abortion or calving area must be disinfected, and effective fencing is required [23].
Mass vaccination is crucial for the control and eradication of bovine, ovine and caprine brucellosis but other complementary measures that may need consideration include improved farm hygiene, restriction and control of trade and movement of animals, testing of animals and isolation and removal of infected animals. Though the existing vaccine for bovine brucellosis, theB. abortusstrain 19 (S19), and the vaccine f or ovine and caprine brucellosis, theB. melitensisstrain Rev 1, have proven to be very useful under most conditions [27].
It is often recommended that vaccination with strains 19 and Rev.1 should be limited to sexually immature female animals. This is to minimize stimulation of post-vaccinal antibodies which may confuse the interpretation of diagnostic tests and also to prevent possible abortion induced by the vaccines [14]. However, field and laboratory studies have demonstrated that conjunctival administration of these vaccines makes the vaccination of the herd or flock a practical and effective procedure. Rapid herd immunity is developed, and application costs are minimized. The lowered dose results in lower antibody titres and these recede rapidly.Several diagnostic tests have been developed which are useful in differentiating antibody classes. Of these, the complement fixation test and ELISA are currently the most widely used [3].
Generally, the following guidelines which should be considered for control of brucellosis: Proper diagnosis; Scheduled vaccination programs for young animals; Screening of herds, livestock markets, abattoirs & subsequent removal of diseased; Awareness among the farmers, livestock & public health authorities(Figure 1) [26]
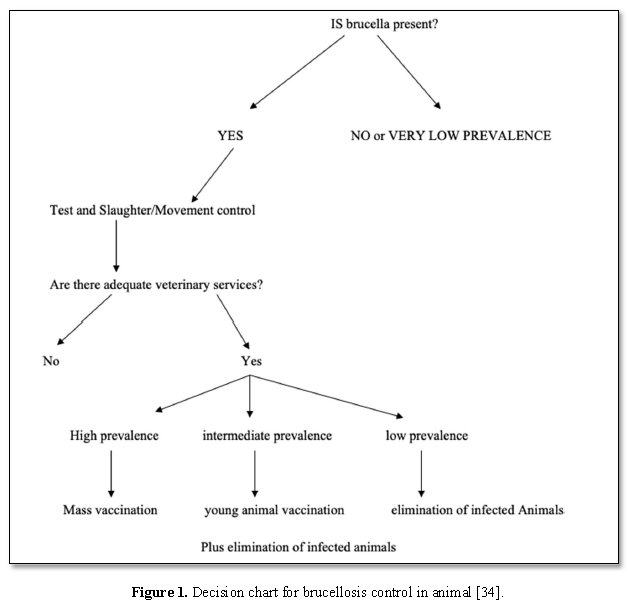

Status of Bovine Brucellosis in Ethiopia
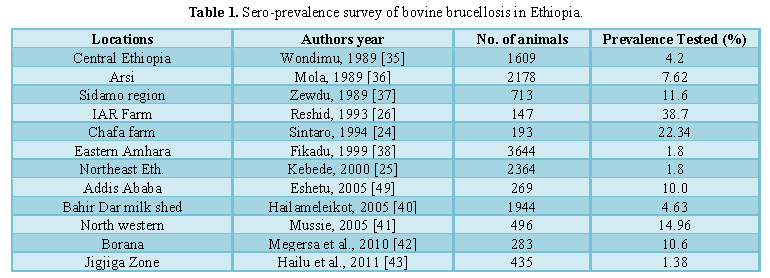
Prevalence: Serological investigation on the prevalence of bovine brucellosis in Ethiopia has been carried out in different parts of the country for the last decades. Several investigators have established the endemicity of bovine brucellosis in different parts of the country and the available information on brucellosis clearly showed that the disease is endemic and widely spread with significant economic and public health importance [6].The importance of Bovine Brucellosis and its prevalence in Ethiopia has been reported by different researchers and it is indicated in Table 1.

Economic importance
Brucellosis occurs worldwide in domestic and game animals and it is one of the major causes of a serious economic problem for the intensive and extensive animal production system of the tropics. In infected cattle populations’bovine brucellosismight leadtoa lower calving rate due to temporary infertility and/or abortion, resulting in a decreased milk production of cows, increased replacement costs as well as lowered sale value of infected cows [44].
Bovine Brucellosis has considerable impact on animal and human healthy as well as a wide socio-economic impact, especially in countries in which rural income relies largely on livestock breeding and dairy product [45]. Studies [44] summarized the economic losses of brucellosis to be: Losses due to abortion in the affected animal population; Diminished milk production, Brucella mastitis and contamination of milk; Cull and condemnation of infected animals due to breeding failure; Endangering animal export trade of a nation; Human brucellosis causing reduced work capacity through sickness of the affected people; Government costs on research and eradication schemes; Losses of financial investments [36].
Most studies that focused on bovine brucellosis in Ethiopia cattle highlight the fact that the control of bovine brucellosis is of economic importance. However, only very few studies were found to have carried out a crude economic analysis to evaluate the impact of bovine brucellosis in traditional cattle production systems, or to evaluate the possible costs of controlling the disease. The economic impact of brucellosis on livestock species can be estimated for direct losses due to morbidity and mortality and indirect losses due to treatment cost [46].
Bovine Brucellosis causes economic losses through abortions, stillbirths or the death of young stock. The disease can also have a blow on exports and have negative impact on the efforts to improve breeding. Brucellosis has a Considerable impact on animal and human health, as well as wide socio-economic impacts, especially in countries in which rural income relies largely on livestock breeding and dairy products including Ethiopia [40]. The economic importance of livestock goes beyond direct food production. Skins, fibbers and manure (fertilizer or fuel) [44].
Public healthy significance of bovine brucellosis
Brucellosis is a significant zoonosis, and health authorities must be alerted to the potential for human infection. The major risk to the general public is from consumption of unpasteurized milk from infected cows. People handling infective material (including vaccines) must be advised of appropriate occupational health and safety requirements [34].
In human, Brucellosis is a multi-systemic disease with a broad spectrum of symptoms. Asymptomatic infections are common. In symptomatic cases, the disease is extremely variable, and the clinical signs may appear insidiously or abruptly. Typically, brucellosis begins as an acute febrile illness with nonspecific flu-like signs such as fever, headache, malaise, back pain, myalgia and generalized aches. Drenching sweats can occur, particularly at night. Splenomegaly, hepatomegaly, coughing and pleuritic chest pain are sometimes seen. Gastrointestinal signs including anorexia, nausea, vomiting, diarrhea and constipation occur frequently in adults but less often in children [7].
Brucellosis in human represents a major public health hazard, which affects social and economic development in various countries.Groups at high risk for brucellosis are animal health workers, butchers, farmers, veterinary clinicians and those who are habitually consume raw milk and come in contact with animals [47].
Person-to-person transmission is not a significant problem except through blood or organ transfer which should be subject to proper control. Airborne or contact of infection through environmental contamination may be a significant problem when infected animals pass through densely occupied areas, e.g. on the way to market, abattoir and laboratory. Appropriate measures should be taken to address these problems. A key means of achieving this is through education of the population, and especially those directly involved in the animal and food industries. All measures should be integrated into adequately designed and effectively implemented control programs. Close collaboration between public health and veterinary services as well as other relevant agencies is fundamental in order to meet the targets [14].
Public health significance includes illness, physical incapacity and loss of manpower and also results in the scarcity of animal proteins due to loss of meat. Occupational risk of brucellosis is important because of the high possibility of direct transmission from infected animals to the people employed in animal husbandry. Herdsmen are the most exposed members. This occupational exposure is high in Ethiopia, where herding of animals is traditional and unscientific [11].
No vaccine is available for the prevention of brucellosis in human. Therefore, preventive measures will be essential to minimize the risk of infection of the human population.Such measures should include improved food hygiene including the pasteurization of milk and protection from infection of high-risk groups such as milkers and other people working in the dairy industry [43].
CONCLUSIONS AND RECOMMENDATIONS
Brucellosis in animals and humans is worldwide distributed and being considered one of the most important zoonosis. The disease is transferred from animals to man. The bacteria multiply in the reproductive organs and mammary glands of infected animals.Infected animals are most contagious when they deliver or abort.Serological investigation on the prevalence of bovine brucellosis in Ethiopia has been carried out in different parts of the country and several investigators have established the endemicity of bovine brucellosis in the Ethiopia. It is difficult to control the disease without having good information about the disease in reservoir animals. Major national socio-economic consequences through very serious international trade losses, national market disruptions and very severe production losses in the livestock industries that are involved. Brucellosis in humans can cause undulant fever, malaise, anorexia, headache, arthralgia, constipation, sexualimpotence, nervousness and depression. In Ethiopia, for instance, human life is highly associated with livestock population in the different livestock production systems and people live very closely with livestock having a high incidence of bovine brucellosis and thus, are at higher risk of acquiring the infection. Thus, there is a need to design and implement control measures aiming at preventing further spread of the disease in the Region through the use of better management practices. In addition, the public in general and high-risk group in particular should be made aware of the zoonotic potential of bovine brucellosis.
Based on the above conclusion the following recommendations are forwarded:
-
Human and animal laboratories facilities need to be supplemented performing all tests related to brucellosis.
-
An improved epidemiological survey is needed to determine the magnitude and distribution of the Brucellosis problem both in humans and animals.
-
Quarantine and control measures should be established.
-
High risk groups and general population should be given health education about the nature of the disease.
-
In infected areas, trade for consumption of fresh milk and dairy products should be strictly controlled and limited to certified Brucellosis farms.
- Kebede T, Ejeta G, Ameni G (2008) Sero-prevalence of bovine brucellosis in small holder farms in central Ethiopia (Wuchale-Jida district). Revue de Medicine Veterinaries 159: 3-9.
- Seifert HSH (1990) Human hygiensiche and wirts chaftliche Bedeutung der Brucellose Inder Tierhaltung des sahel. Gottinger Beitr Land Forstwirt. Trop Subtrop 51: 187-192.
- FAO (2006) Brucellosis in humans and animals, United Nation, pp: 39.
- Crawford RP, Huber JD, Adams BS (1990) Epidemiology and surveillance. Animal brucellosis, pp: 131-151.
- Asfaw Y, Molla B, Zessin HK, Tegene A (1998) The epidemiology of bovine Brucellosis in intra and peri-urban dairy production systems in and around Addis Ababa. Bull Anim Hlth Prod Afr 46: 217-224.
- Kamil K (2011) Sero-prevalence study of Bovine Brucellosis in pastoral and agro- pastoral herds of Cattle in and around Yabello District and its Zoonotic implication, Southern Ethiopia, DVM Thesis, College of Agriculture and Veterinary Medcine, Jima University, Ethiopia.
- Abbas B, Agab H (2002) A review of camel brucellosis. Prev Vet Med 55: 47-56.
- Than NT (2007) Prevalence survey of Bovine Brucellosis (Brucella abortus) in Dairy Cattlein Yangon, Myanmar, pp: 13.
- Grimont F, Verger JM, Corneils P (1992) Molecular typing of Brucella with cloned DNA probes. Res Microbiol 143: 55-65.
- Charters AD (1980) Brucellosis. Austr Fam Physician 9: 707-712.
- Jiksa K (2003) Sero-epidemiological Study of Brucellosis in Humans and Dairy Cattle. MSc Thesis FVM, Addis Ababa University, Ethiopia.
- Corbel MJ (1997) Brucellosis: An overview. Emerg Infect Dis 3: 213-221.
- Dermott JJM, Arimi SM (2002) Brucellosis in sub-Saharan Africa: Epidemiology, control and impact. Vet Microbiol 90: 111-134.
- OIE (2009) Terrestrial Animal Health Code Brucellosis. Available online at: http://www.oie.int/
- Mohammad A, Mehwish M, Muhammad JA (2011) Bovine Brucellosis: Old and new concepts. PVJ(PRINT): 2074-7764.
- Nicoletti P (1980) The epidemiology of bovine brucellosis. Advance in Vet Sci Compar Medicine 24: 69-98.
- Tolosa T (2004) Sero-prevalence stud of bovine brucellosis and its public health significance in selected sites of Jimma zone, Western Ethiopia. MSc thesis, FVM, AAU, Debre Zeit, Ethiopia.
- Radostits OM, Gay CC, Blood CD, Hinchelclif KW (2000) Veterinary Medicine: A textbook of the diseases of cattle, sheep, pigs, goats and horses. 9th ed., 867-882.
- Hussein AA, Assayed A, Feki M (2005) Sero-epidemiological study on human brucellosis in Assiut Governorate. Egy J Immunol 12: 49-56.
- Enright FM (1990) The pathogenesis and pathobiology of Brucella infection in domestic animals. Animal Brucellosis, pp: 301-320.
- Rodostits M, Gay C, Hinchcliff W, Constable D (2007) Veterinary Medicine. A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10th edition Grafos, S.A. Arte sobrepapel, Spain.
- DEFRA (2002) Department for Environment, Food & Rural Affairs (DEFRA): Notifiable diseases, disease information-Brucellosis (Brucella abortus).
- AUSVETEP (Austrian Veterinary Emergency Plan) (2005) Disease Strategy Bovine brucellosis Version 3.0, pp: 8-10.
- Sintaro T (1994) The impact of brucellosis on productivity in an improved dairy herd of Chaffe state farm, Ethiopia. MSc thesis, Faculty of Veterinary Medicine, Frei university, Berlin.
- Kebede F (2000) An epidemiological survey of bovine brucellosis in northeastern Ethiopia. Proceedings of the 11th Annual Conference of Ethiopian Veterinary Association. June 8-9, 2000. Addis Ababa, Ethiopia: pp: 1-43.
- Rashid M (1993) Reproductive wastage in cattle due to bovine brucellosis. Institute of Agricultural Research: Proceedings of the Fourth National Livestock Improvement Conference, held at Addis Ababa, 13-15th November, 1993, pp: 270-272.
- Schurig GG, Sriranganathan N, Corbel MJ (2002) Brucellosis vaccines: Past, present and future. Vet Microbiol 90: 479-969.
- Geering WA, Forman AJ, Nunn MJ (1995) Exotic diseases of animals: A field guide for-australian veterinarians. Australian Government Publishing Service Canberra, pp: 22-33.
- Radwan AI, Bekairi SI, Bokmy AMA, Prasad PV, Mohammad OM, Hussein ST (1993) Successful therapeutic regimens for treating Brucella melitensis and Brucella abortus infections in cows. Review Sci Technol 12: 909-922.
- Nielsen, K (2002) Diagnosis of brucellosis by serology. Vet Microbiol 90: 447-459.
- Abreha T (2003) Brucellosis in cattle and small ruminants in selected sites of Tigray Region, North Ethiopia. DVM. Thesis, FVM, AAU, Debre-Zeit, Ethiopia.
- Luzzi GA, Brindle R, Sockett PN, Solera J, Klenerman P, et al. (1993) Brucellosis imported and laboratory acquired cases: An over view of treatment trials. Trans R Soc Trop Med Hyg 87: 138-141.
- Henk L, Smits S, Kadri M (2004) Brucellosis in India: A deceptive infectious disease, pp: 2-3.
- WHO (1998) Human and animal brucellosis. Report of WHO workshop, Damascus. Syria Arab republic.
- Wondimu A (1989) The epidemiology and economic of bovine brucellosis in the central high land of Ethiopia. MSc Thesis, Veru, Reading Univ.
- Molla B (1989) Sero-epidemilogical survey of bovine brucellosis in Arsi region. DVM Thesis, FVM, AAU Debre Zeit, Ethiopia.
- Zewdu E (1989) Seroprevalence Study of Bovine Brucellosis in Selected Sites of Sidamo Region. DVM Thesis, Faculty of Vet Med, Addis Ababa Univ, Debre Zeit, Ethiopia.
- Fikadu K (1999) An epidemiological survey of Bovine Brucellosis in Amhara National Regional State (unpublished).
- Eshetu Y, Kassahun J, Abebe P, Beyene M, Zewdie B, et al. (2005) Sero-prevalence study of brucellosis on dairy cattle in Addis Ababa. Bull Anim Health Product Africa 53: 211-214.
- Hailemelekot M (2005) Sero-prevalence study of brucellosis in cattle and human in Bahirdar milk shed, FVM, AAU, Debre Zeit, Master’s Thesis.
- Mussie H (2005) Ser o-prevalence study of brucellosis in cattle and human in Bahirdar milk shed. MSc. Thesis, FVM, AAU, Debre Zeit, Ethiopia.
- Megersa B, Biffa D, Abunna F, Regassa A, Godfroid J, et al. (2010) Seroprevalence of brucellosis and its contribution to abortion in cattle, camel and goat kept under pastor al management in Borana, Ethiopia, pp: 55-86.
- Hailu D, Mohamed M, Mussie H, Moti Y (2011) Seroprevalence of bovine brucellosis in agro pastoral areas of Jigjiga zone of Somali National Regional State, Eastern Ethiopia. 15: 37-47.
- Mangen M, Otte M, Pfeiffer J, Chilonda P (2002) Bovine brucellosis in Sub-Sahara Africa: Estimation of sero-prevalence and impact on meat and milk off take potential. Food and Agriculture Organization Livestock Information and Policy Branch, pp: 32-58.
- Dermott JJM, Deng KA, Jayatilka TN, Jack MA (1987) A cross sectional Cattle disease study in Kongorrural council. II Brucellosis in cows: Associated Factors impact on production and disease control considerations. Prev Vet Med 5: 125-132.
- Asmare K (2004) Epidemiology of brucellosis in cattle and its sero-prevalence in animal Health professionals in Sidama zone, Southern Ethiopia. MSc Thesis, FVM, AAU, Debre Zeit, Ethiopia.
- Radostits OM, Blood DC, Gay CC (1994) Veterinary Medicine: Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses. 8th edition, London: Bailliere Tindall, pp: 787-792.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Journal of Microbiology and Microbial Infections (ISSN: 2689-7660)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Advances in Nanomedicine and Nanotechnology Research (ISSN: 2688-5476)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Astronomy and Space Research
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)



