3917
Views & Citations2917
Likes & Shares
INTRODUCTION
Gentamicin sulphate, an aminoglycoside broad spectrum anti-bacterial, belongs to class III of Biopharmaceutics Classification Systems (BCS). It is highly water soluble but lack intestinal permeability. Gentamicin sulphate is a complex of three major and several minor components with gentamicins C1, C1a and C2 being the three major components of the drug complex. The C2 component consists of two stereoisomers (C2 and C2a). However, the component ratio in commercial gentamicin sulphate preparations varies remarkably and is generally unknown [1].
Various formulations of the drug exist. Conventionally, gentamicin sulphate is administered parentally [2,3]. The drug has also been presented for dermal applications [2,4-7] and as inhalations [3,8-10]. It has been evaluated for rectal local activity [11] as well as implants [12,13]. Attempts have been made to formulate the drug for oral administration by incorporating intestinal mucosa penetration/permeation enhancers to achieve systemic circulation [14-16]. Umeyor et al [17] have achieved improved systemic circulation of orally delivered gentamicin sulphate by surface modified self-nanoemulsifying formulations (SNEFs). Adopting these novel approaches to conventional tableting and capsulations technologies may present fresh problems of cost effectiveness among other difficulties. Nwakile et al [18] have indicated that an unaided formulation of the drug administered orally will invariably accumulate in the gut and the drug’s broad-spectrum antibacterial activity could be of use for local action against susceptible bacteria pathogens inhabiting the GIT. Presenting gentamicin sulphate as an oral solid dosage form requires granulation. Dry granulation/slurring may not favor the manufacturing processes of the gentamicin sulphate oral solid dosage form because of its hygroscopic nature which can lead to moisture absorption from the environment. Wet granulation of gentamicin sulphate involving water is not plausible because of similar reason. In this research therefore, PEG 4000 was employed as a non-aqueous granulating aid to granulate gentamicin sulphate into granules in the presence of the following excipients; Carbisol®, N-modified starch and Ac-di-sol®. The granules were packaged into gelatine capsules. The drug contents of the capsule dosage form were monitored for possible drug-excipient interaction for a period of eight weeks.
MATERIALS AND METHODS
Formulation of different batches of the gentamicin sulphate granules
The formula used by Nwakile et al [18] for-gentamicin sulphate granules formulation as contained in Table 1 was employed. The granulating aid (PEG 4000) was weighed out into a beaker and melted on a hot plate. The diluent (N-starch), the desiccant (Carb-o-sil®) and the disintegrate (Ac-di-sol®) were weighed out and thoroughly mixed. The mixture was then dispersed into the melted PEG 4000 and thoroughly mixed. The beaker was removed from the hot plate and the gentamicin sulphate powder introduced. The mixture was thoroughly mixed and allowed to cool and the powder formed a firm dry lump. The lumps were forced through a 0.8 mm mesh to obtain the granules. The granules were packed into a transparent wide mouthed glass container wrapped with aluminium foil and stored in a desiccator for further use.
Micromeritic studies
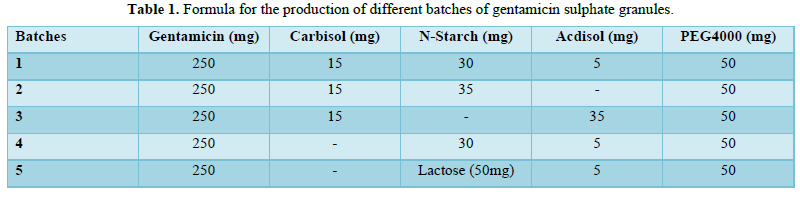
Bulk density studies
The bulk density of each granule was obtained by weighing a 10 g sample and gently poured into a 50 ml cylinder. The volume (Vb) occupied by the sample mass (M) was recorded. The bulk density was calculated using equation 1. The study was carried out in triplicate.
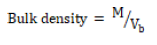 (1)
(1)
Tap density studies
A 10 g quantity of each granule samples were weighed gently poured into 50 ml cylinder and leveled without compaction. The cylinder was tapped gently onto a soft surface from a height of 4 cm at two second interval. Tapping was continued until no further change in volume was noted. The volume of the tapped sample (Vt) was recorded and the tap density calculated using equation 2. The study was carried out in triplicate.
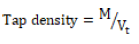 (2)
(2)
Angle of repose studies
The angles of repose of the granules were obtained by the fixed funnel method. The funnel was arranged in a manner that the funnel tip was 5 cm from the platform. Through the funnel, the sample was allowed to flow onto the surface of the platform. The height (h) and radius (r) of the pile were measured. The test was carried out in triplicate. Angle of repose was calculated using equation 3.
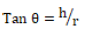 (3)
(3)
Granules flow rate
Granules flow rate was studied directly by noting the rate at which the granules were discharged from a container through holes and tubes. A 50 g granule was allowed to fall from a known height through a funnel to which a discharge tube was attached. The time (t) taken for the granule mass (M) to flow through the channel was noted. The flow rate was thereafter deduced using equation 4.
 (4)
(4)
Capsule filling
The gentamicin sulphate granules were manually filled into transparent gelatine capsules of size 00 based on each batch total weight as contained in Table 1.
Establishment of calibration curve for gentamicin sulphate using S. aureus
The calibration curve was established using solution of gentamicin sulphate with the following concentrations: 100-, 50-, 25-, 12.5-, 6.25-, and 3.125- µg/ml. A culture of Staphylococcus aureus obtained from the laboratory stock of the Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences Nnamdi Azikiwe University Awka, Anambra State Nigeria was used for the in-vitro bioassay. Twenty (20) ml of molten Mueller-Hinton agar was poured into sterile Petri dishes and standardized concentrations (McFarland 0.5) of overnight culture of the selected organism (S. aureus) introduced aseptically on the agar plates. The mixtures were gently rocked and allowed to solidify. Holes of 5 mm diameter were made in the agar plates using a sterile metal cork-borer. A 20 µl of the various concentrations of the drug were put in each hole under aseptic condition and kept at room temperature for 1 h to allow for diffusion. The plates were then incubated at 37°C for 24 h and the inhibition zone diameters (IZDs) measured and recorded. The size of the cork borer (5 mm) was deducted from the values recorded for the IZDs to get the actual diameter. This procedure was conducted in triplicate and the mean IZDs calculated and recorded. Thereafter, the IZDs were plotted against the logarithm of the concentrations.
Dissolution studies of gentamicin sulphate capsules
The dissolution was done using BP basket method. Normal saline was used as the dissolution medium. A 300 ml of the saline solution was introduced into a 1000 ml beaker mounted on a hot plate-magnetic stirrer. The solution was maintained at a temperature of 37 ± 0.5°C and speed of 100 rpm. The capsule contained in a stainless-steel basket was suspended unto the medium using retort stand. The dissolution was allowed to run for 60 min. A 2 ml of the sink was collected at 5 min interval with replacement. The collected sink was used to inoculate the S. aureus seeded Mueller-Hinton agar.
Actual concentrations of drug in the sinks were obtained by superimposing the IZD of the sink on the IZD axis of the calibration curve and extrapolating to the log concentration. The concentration was then obtained by taking the antilogarithm of the log concentration.
Ex vivo drug assay of the gentamicin sulphate capsules
Male and non-pregnant female albino Wistar rats weighing between 155-300 g were used for the study. A culture of S. aureus was used. Gentamicin sulphate granules obtained from the capsules and dissolved in 2 ml of water was administered orally via oral intubation at the dose level of 14.3 mg/kg to the corresponding group of two rats per group for the batches. The number of rats was deliberately made small (2 rats), to enable utilization of the entire droppings produced by the group in a particular time interval. All the rat droppings were collected at 0-, 1-, 2-, 4- and 6- h time interval. A 10 % mixture of the dropping was made in distilled water and centrifuged after agitation for 10 min. Centrifugation was done at 4000 rpm for 10 min. A 20 µl of the decant was introduced into a 5 mm hole bored in a solidified Mueller-Hinton agar seeded with the S. aureus and incubated for 24 h at 37°C. Zones of inhibition were measured planimetrically. Actual concentrations of drug in the specimen were obtained by superimposing the IZD of the filtrate on the IZD axis of the calibration curve and extrapolating to the log concentration. The concentration was then obtained by taking the antilogarithm of the log concentration.
Differential Scanning Calorimetry (DSC) studies
Differential scanning calorimeter (DSC 822e, Mettler Toledo, Greifensee, Switzerland & Columbus, Ohio, USA) equipped with STARe software was employed for DSC study of the granules, drug (gentamicin sulphate), and the excipients; PEG 4000, N-modified starch, Carb-o-sil® and the Ac-di-sol®. A 5 mg of the granules or ingredients were weighed into standard aluminium pan. Samples were hermetically sealed and equilibrated for 24 h at room temperature before heat treatment in the DSC apparatus. The samples were heated at a rate of 10°C/min from 25 to 450°C. After heat treatment, samples were cooled to 25°C and removed from DSC.
Statistical analysis
Stoicastic statistics of mean and standard deviation were employed where appropriate.
RESULTS AND DISCUSSION
It has been shown that gentamicin sulphate when administered orally at low doses does not permeate into the systemic circulation [18]. This means that the drug will be localized in the GIT. Localization of the drug will invariably inhibit sensitive pathogenic bacteria inhabiting the region. The success of such localized treatment will definitely depend on the availability of suitable dosage forms. It has been shown that at plausible formulation dose ranges of 1000mg/70kg, 750mg/70kg and 500mg/70kg administered orally at 8 h intervals, gentamicin sulphate could be used for such localized treatment [18].
Formulation of oral solid dosage form of gentamicin sulphate was achieved with the formula as shown in Table 1. The granules physically appeared gritty to touch and fairly hard. The ability of the granules to flow well during tableting/capsulation was assessed by their micromeritic parameters which predict the flow pattern of such materials. Material particles with Hausners’ quotient of 1.2 and minimum angle of repose of 25o indicate excellent flow [19]. To access this ability of the granules to flow well, some of the flow indicators were evaluated. From the result of the micromeritic studies as shown in Table 2, the values of the three flow indicators assessed; Hausners’ quotient, angle of repose and flow rate indicated that the granules have excellent flow properties.
Dissolution studies of gentamicin sulphate capsules
The ability of the formulated capsule dosage form to release the drug content was assessed through dissolution studies. The sink arising from such dissolution was used to inoculate an agar seeded with S. aureus. The zones of inhibition so obtained were superimposed on the gentamicin sulphate calibration shown in Figure 1.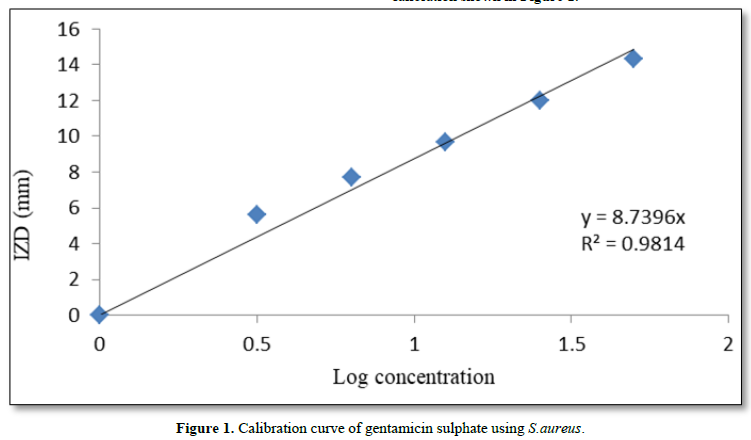
The actual concentrations of the gentamicin sulphate released from the capsules over a period of 60 min were used to construct the concentration-time release curve. Such release studies done over a period of seven weeks post formulation were presented as shown in Figures 2a-c. It is evident from the figures that the formulated capsules retained and released the drug content up to three weeks post formulation.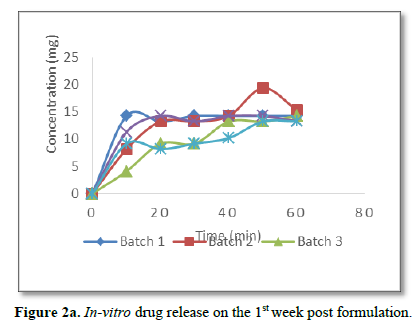
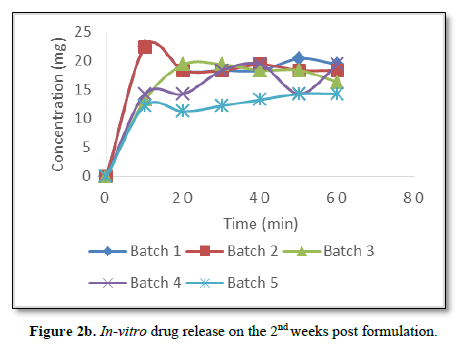
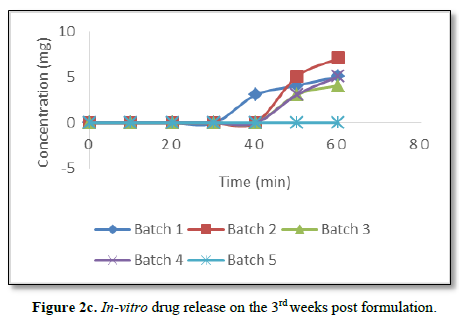
Significantly, there was delay of drug release from the capsules after the 3rd week post formulation as can be seen from Figure 2c. There was no drug release from batch 5 while other batches started releasing their drug content after 20 min of dissolution.
Significantly, dissolution studies on the batches from the fourth week till the seventh week post formulation and inoculation of the sink unto the S. aureus seeded agar failed to show any activity. This means that the potencies of all the batches of the gentamicin sulphate capsules were lost in-vitro from the fourth week post formulation.
However, to ascertain if such loss of activity is a reversible or destructive/permanent interaction, capsules from the batches stored for 8 weeks were reconstituted with distilled water and administered to the rats at 14.3 mg/kg. The filtrates from a 10 % reconstitution of the rats’ droppings were inoculated unto the S. aureus seeded agar. As can be seen from Figure 3, there are periodic release in-vivo of the active drug. These periodic releases imply that the active drug is being release at different intervals from the complexes formed with the respective ingredient(s).
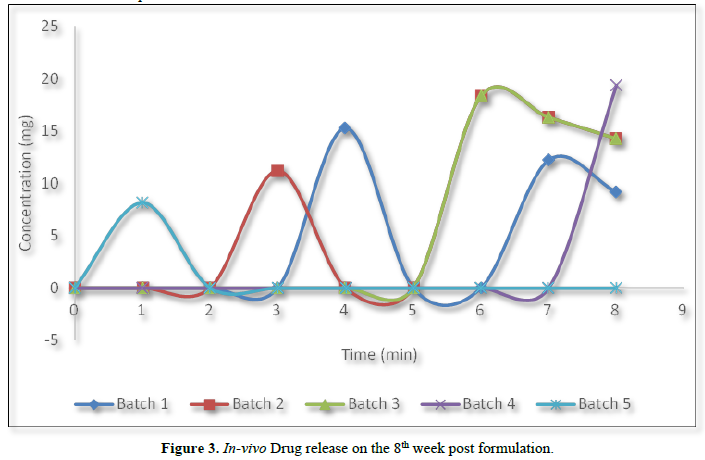
All the batches except batch 5 regained activity following oral administration to the rats. Non-restoration of anti-bacterial activity as observed in batch 5 implies that the ingredients in the formulated batch must have interacted destructively with the drug. As can be seen from Table 1, batch 5 differs from other batches by having lactose as the diluents. The destructive interaction as observed in batch 5 can only be attributed to the presence of this lactose.
Gentamicin sulphate by its structure is a highly charged hydrophilic compound, it tends to complex with the excipients in the formulation which leads to loss of its antibacterial activity in-vitro after three weeks of formulation, but on reaching the GIT, the complexes of the drug revert back generating active drug (gentamicin sulphate) to elicit anti-bacterial activity.
Significantly, the adjuvant that is responsible for these reversible interactions can be ascertained by observing the DSC thermograph of the adjuvants singly and in combination with the drug as obtained in the formed granules.
To this end and following DSC studies as shown in Figures 4A-F, gentamicin sulphate peaks at 245.09 and 286.33. The Carbisol® used as desiccant peaks at 93.94 while N-starch, a novel modified maize starch with hydrophobic character, used as diluent peaks at 85.96 and 262.40. Ac-di-sol® used as disintegrate peaks at 161.50 and 234.80 while PEG 4000 that serves as the granulating aid peaks at 63.05. Surprisingly, all these peaks disappeared as the drug and the excipients interacted in the formed granules, new peaks of 63.88 and 302.85 appearing on the granules as shown in Figure 4F.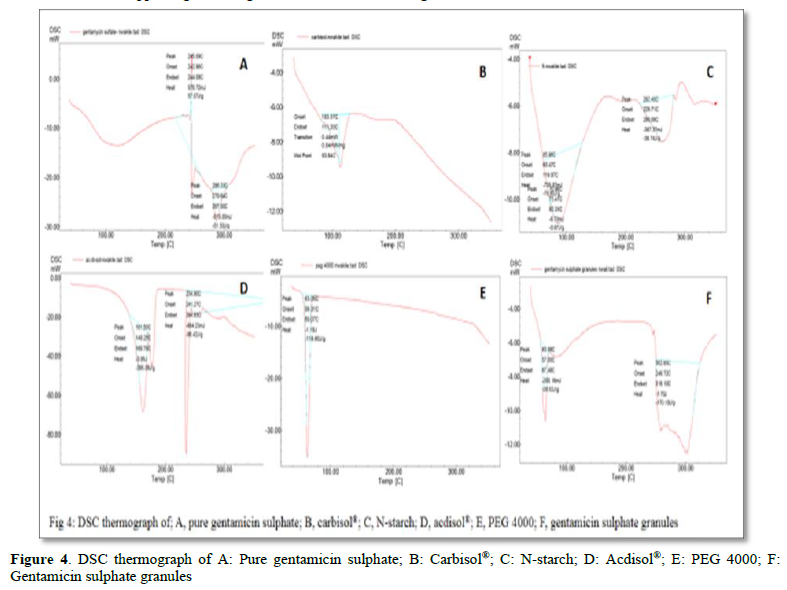
The implication is that granulation of gentamicin sulphate with the excipients leads to complexation and the complexes failed to elicit antibacterial activity in-vitro as from the fourth week post formulation. Most of these complexes are reversible in-vivo, which gives rise to pro-drug of gentamicin sulphate. Pro-drugs have been described as bio reversible derivatives of drug molecules that must undergo in vivo biotransformation to release the active drug by chemical or enzymatic cleavages to exert its desired pharmacological effect [20,21].
CONCLUSION
Formulation of gentamicin sulphate into capsule leads to pro-drug formation. The pro-drug formed from the drug-excipients interaction can be utilized in the treatment of susceptible bacteria inhibiting the GIT.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interest: None.
- Claes PJ, Busson R, Vanderhaeghe H (1984) Determination of the component ratio of commercial gentamicins by high-performance liquid chromatography using pre-column derivatization. J Chromatograph A 298: 445-457.
- Imamura S, Adams JC (2003) Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol 4: 176-195.
- Labiris NRC, Holbrook AM, Chrystyn H, Macleod SM, Newhouse MT (1999) Dry powder versus intravenous and nebulized gentamicin in cystic fibrosis and bronchiectasis; a pilot study. Am J Respir Critical Care Med 160: 1711-1716.
- Ipsen T, Jørgensen PS, Damholt V, Tørholm C (1991) Gentamicin-collagen sponge for local applications: 10 cases of chronic osteomyelitis followed for 1 year. Acta Orthopaedica Scandinavica 62: 592-594.
- Nnamani PO, Kenechukwu FC, Dibua EU, Ogbonna CC, Momoh MA, et al. (2013) Formulation, characterization and ex-vivo permeation studies on gentamicin-loaded transdermal patches based on PURASORB® polymers. Sci Res Essay 8: 973-982.
- Pick J, Olson LL, Ellis WY, Lim P (1997) Development and validation of a method to extract and quantitate paromomycin and gentamicin from an Aquaphilic® cream formulation. J Pharm Biomed Anal 16(1): 131-137.
- Ravis WR, Llanos-Cuentas A, Sosa N, Kreishman-Deitrick M, Kopydlowski KM, et al (2013) Pharmacokinetics and absorption of paromomycin and gentamicin from topical creams used to treat cutaneous leishmaniasis. Antimicrob Agents Chemother 57: 4809-4815.
- Aquino RP, Prota L, Auriemma G, Santoro A, Mencherini T, et al. (2012) Dry powder inhalers of gentamicin and leucine: Formulation parameters, aerosol performance and in vitro toxicity on CuFi1 cells. Int J Pharm 426: 100-107.
- Goldman JM, Bayston SM, O'Connor S, Meigh (1990) Inhaled micronized gentamicin powder: A new delivery system. Thorax 45: 939-940.
- Lim ST, Forbes B, Berry DJ, Martin GP, Brown MD (2002) In vivo evaluation of novel hyaluronan/chitosan microparticulate delivery systems for the nasal delivery of gentamicin in rabbits. Int J Pharm 231: 73-82.
- Fix JA, Patricia A, Porter PA, Leppert PS (1983) Involvement of active sodium transport in the rectal absorption of gentamicin sulfate in the presence and absence of absorption-promoting adjuvants. J Pharm Sci 72: 698-700.
- Krasko MY, Golenser J, Nyska A, Nyska M, Brin YS, et al. (2006) Gentamicin extended release from an injectable polymeric implants. J Control Release 117: 90-96.
- Soriano I, Evora C (2000) Formulation of calcium phosphates /poly (d,l-lactide) blends containing gentamicin for bone implantation. J Control Release 68: 121-134.
- Anilkumar P, Badarinath, AV, Naveen N, Prasad K, Reddy BRS, et al. (2011) A rationalized description on study of intestinal barrier, drug permeability and permeation enhancers. J Global Trend Pharm Sci 2(4): 431-449.
- Rama Prasad YV, Eaimtrakarn S, Ishida M, Kusawake Y, Tawa R, et al. (2003) Evaluation of oral formulations of gentamicin containing labrasol in beagle dogs. Int J Pharm 268: 13-21.
- Shaikh MS, Derle ND, Bhamber R. (2012) Permeability enhancement techniques for poorlypermeable drugs: A review. J Appl Pharm Sci 02(06): 34-39.
- Umeyor C, Attama A, Uronnachi E, Kenechukwu F, Nwakile C, et al. (2016). Formulation design and in vitro physicochemical characterization of surface modified self-nanoemulsifying formulations (SNEFs) of gentamicin. Int J Pharm 497: 161-198.
- Nwakile Dozie C, Dozie-Nwakile OC, Okoye EI, Umeyor CE, Uronnachi EC, et al. (2021) Non-Absorbable Oral Gentamicin Sulphate: Biopharmaceutical and Dosage Form Evaluation. Eur Pharm J 68(2): 8-15.
- Staniforth JN (1999) Powder flow. In: Aulton, editor. Pharmaceutics, the science of dosage form design. Edinburgh: Churchill Livingstone. 1999: 600-615.
- Cho S, Yoon YR (2018) Understanding the pharmacokinetics of prodrug and metabolite. Transl Clin Pharmacol 26(1): 1-5.
- Markovic M, Ben-Shabat S, Dahan A (2020) Prodrugs for Improved Drug Delivery: Lessons Learned from Recently Developed and Marketed Products. Pharmaceutics 12(11): 1031.








