4504
Views & Citations3504
Likes & Shares
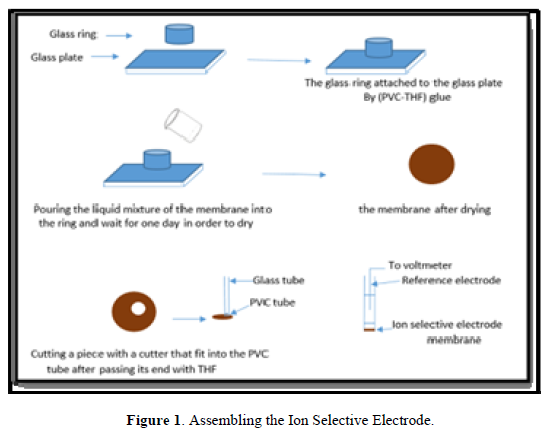
RESULT AND DISCUSSION
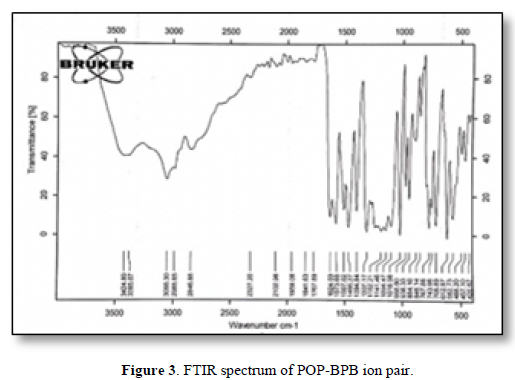
New membranes were prepared for the determination of POP in the pharmaceutical preparations and human fluids, the characterization of these electrodes based on the ion-pair using different plasticizers were described. The new complex (POP-BPB) was proved by the FTIR spectrum as shown in Figures 2 & 3.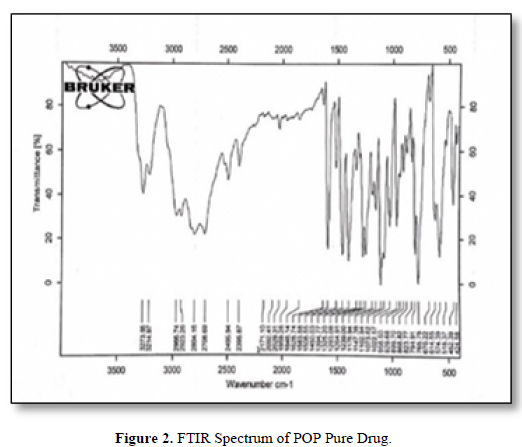
Influence of plasticizers
The effect of the plasticizers on the POP electrode’s response was studied by using three plasticizers which are: Di-butylphthalate (DBPH), Tris (2-ethylhexyl) phosphate (TEHP) and Ortho-nitro phenyl- octylether (ONPOE).
Plasticizers were added to dissolve the ion-pair complex and to set the permittivity of the membrane and the mobility of the ion-exchanger in order to give the prepared electrode, the best selectivity and sensitivity [12].
First electrode for POP drug
This electrode consisted of (POP-BPB) as ion pair, DBPH as plasticizer and PVC as supported which dissolved in THF solvent. The calibration curve for the first electrode was plotted between concentrations range (1×10-6-1×10-2) M of POP drug and the measured potential for each concentration. The statistical treatments of the measured potential are listed in Table 1.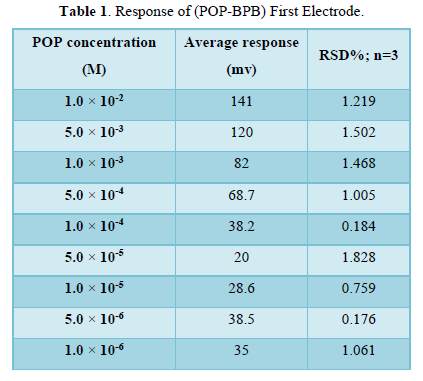
Second electrode for POP drug
The second electrode was prepared from (POP-BPB) as ion pair, TEHP as plasticizer and PVC as supported which dissolved in THF solvent. The calibration curve for the second electrode was plotted between concentrations range (1×10-6-1×10-2) M of POP drug and the measured potential for each concentration. The statistical treatments of the measured potential are listed in Table 2.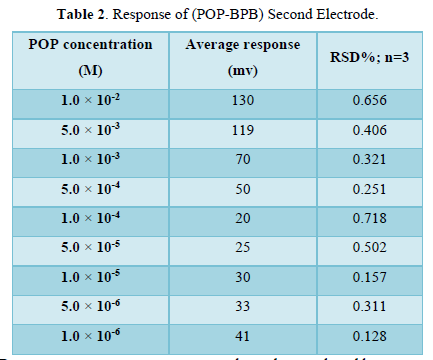
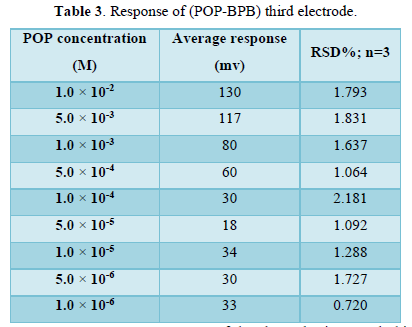
Third Electrode for POP Drug
This electrode was synthesized from (POP-BPB) as ion pair, ONPOE as plasticizer and PVC as supported which dissolved in THF solvent. The calibration curve for the third electrode was plotted between concentrations range (1×10-6 -1×10-2) M of POP drug and the measured potential for each concentration. The statistical treatments of the measured potential are tabulated in Table 3.
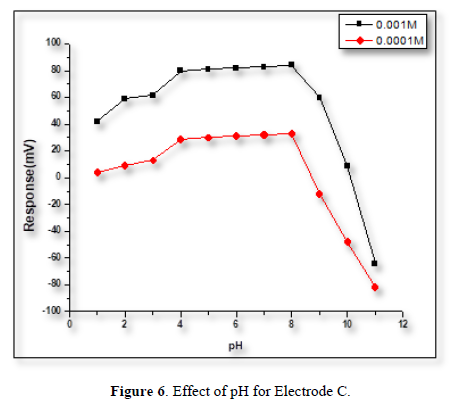
Influence of pH
The effect of pH on the electrode’s potential was evaluated by measuring the potential of the cell at the conc. of (1×10-4 and 1×10-3) M of POP solution. The adjustment of the pH was made by adding some drops of (0.1) M hydrochloric acid or sodium hydroxide. At the low pH level, the potential of the electrodes increased, this is because the electrode has responded to the H+ ions. While at high pH levels, the potential of the electrodes dropped sharply because of the poisoning of the membrane by the formation of the white precipitation. From Figures 4-6 it can be noticed that POP electrodes do not respond to pH changes in the range [4-8].
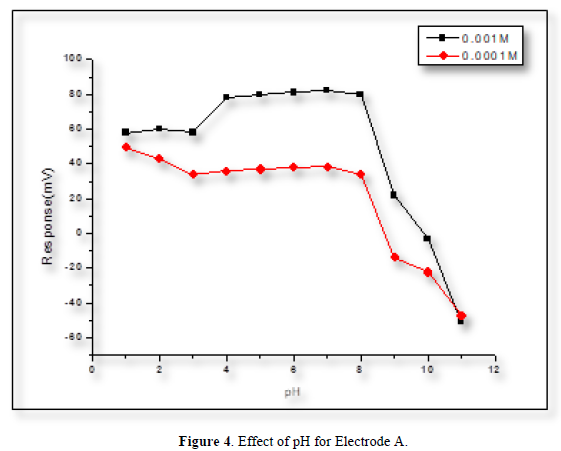
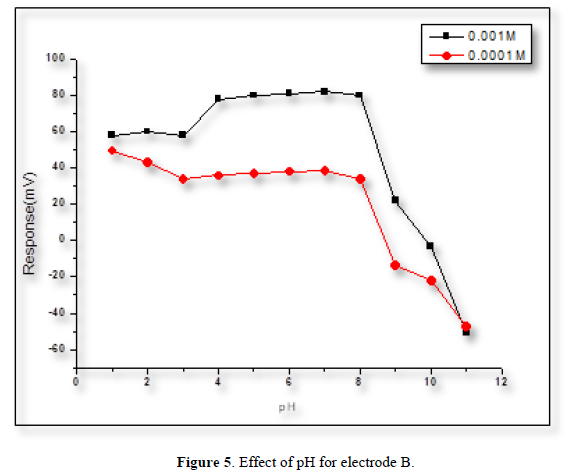
Response time and life time
The response time of the electrode can be defined as the time required for the electrode to reach a stable potential after immersing the proposed electrode and the reference electrode in (1×10-6 and 1×10-2) M of POP solution. The proposed electrodes gave steady potentials after (1), (0.8) and (0.9) min at (1×10-2) M and after (3), (3.8) and (4.5) min at (1×10-6) M for electrode A, B and C respectively. The lifetime of the three electrodes was measured and it was (21), (42) and (1) day for electrode A, B and C respectively. After this time the slope tends to be decreased and the detection limit tends to be increased because of the leaching of the ion pair from the membrane to the external solution (Table 4).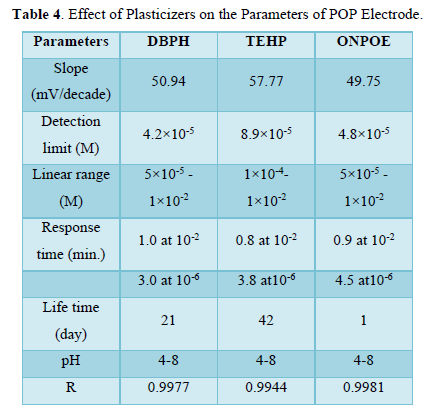
Selectivity
The interferences of some inorganic cations such as: (Li+, Na+, K+, Mg+2, Ca+2, Zn+2, Al+3, Cr+3 and Fe+3) were studied using the separate solution method (SSM) and the Match potential method (MPM). The selectivity coefficient values by (SSM) were calculated by Nickolsky-Eisenman equation and tabulated in Table 5.
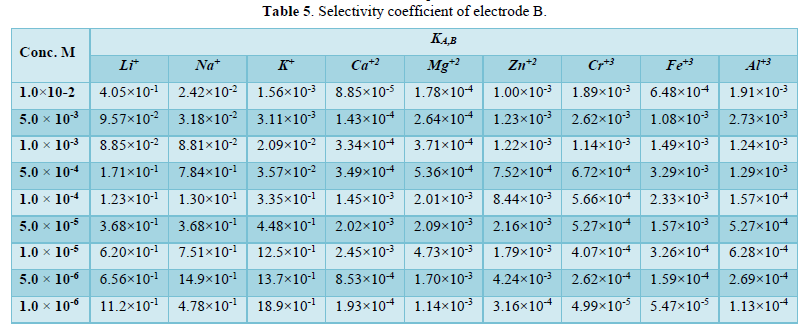
Low values of selectivity coefficients were obtained which means no interfering of these cations on the electrode B response.
CONCLUSION
In this study, the constructions of new three electrodes of POP were based on poly (vinyl chloride) (PVC) membrane. It contains the ion-exchanger that formed between POP and BPB, using three different plasticizers: (DBPH), (TEHP) and (ONPOE). It was a sensitive, precise, rapid and inexpensive method which was used in the determination of POP in the pure form, pharmaceutical preparations and human fluids. The electrode B has shown a good performance with the time stability up to (42) days.
- Gowda BG, Seetharamappa J, Melwanki MB (2002) Indirect spectrophotometric determination of propranolol hydrochloride and piroxicam in pure and pharmaceutical formulations. Anal Sci 18: 671-674.
- Zhang F, Du Y, Ye B, Li P (2007) Study on the interaction between the chiral drug of propranolol and α1-acid glycoprotein by fluorescence spectrophotometry. J Photochem Photobiol B Biol 86: 246-251.
- Rapado-Martinez I, Garcia-Alvarez-Coque M, Villanueva-Camanas R (1997) Liquid chromatographic procedure for the evaluation of β-blockers in pharmaceuticals using hybrid micellar mobile phases. J Chromatogr A 765: 221-231.
- El-Saharty Y (2003) Simultaneous high-performance liquid chromatographic assay of furosemide and propranolol HCL and its application in a pharmacokinetic study. J Pharm Biomed Anal 33: 699-709.
- Partani P, Modhave Y, Gurule S, Khuroo A, Monif T (2009) Simultaneous determination of propranolol and 4-hydroxy propranolol in human plasma by solid phase extraction and liquid chromatography/electrospray tandem mass spectrometry. J Pharm Biomed Anal 50: 966-976.
- De La Pena AM, Salinas F, Duran M (1991) Simultaneous determination of propranolol and hydralazine by derivative synchronous spectrofluorimetry. Analytica Chimica Acta 255: 317-323.
- Ruiz TP, Martı́nez-Lozano C, Tomás V, Carpena J (1998) Simultaneous determination of propranolol and pindolol by synchronous spectrofluorimetry. Talanta 45: 969-976.
- Ramesh K, Gowda B, Seetharamappa J, Keshavayya J (2003) Indirect spectrofluorimetric determination of piroxicam and propranolol hydrochloride in bulk and pharmaceutical preparations. J Anal Chem 58: 933-936.
- Tabrizi AB (2007) A simple spectrofluorimetric method for determination of piroxicam and propranolol in pharmaceutical preparations. J Food Drug Anal 15: 242-248.
- Townshend A, Pulgarın JM, Pardo MA (2003) Flow injection-chemiluminescence determination of propranolol in pharmaceutical preparations. Analytica Chimica Acta 488: 81-88.
- Tsogas GZ, Stergiou DV, Vlessidis AG, Evmiridis NP (2005) Development of a sensitive flow injection-chemiluminescence detection method for the indirect determination of propranolol. Analytica Chimica Acta 541: 149-155.
- Faridbod F, Ganjali MR, Dinarvand R, Norouzi P (2007) The fabrication of potentiometric membrane sensors and their applications. Afr J Biotechnol 6: 2960-2987.








