Research Article
Combination of Percutaneous Kyphoplasty and Zoledronic Acid in Treating Osteoporotic Vertebral Compression Fracture in T2DM Patients
4542
Views & Citations3542
Likes & Shares
Background: Type 2 diabetes mellitus (T2DM) patients maybe have higher risk of new vertebral fracture (NVF) after percutaneous kyphoplasty (PKP) and it remains unknow whether anti-osteoporosis treatment could reduce the NVF.
Objective: This study aimed to investigate the efficacy of combination of PKP and zoledronic acid in diabetic patients with osteoporotic vertebral compression fracture (OVCF).
Methods: 152 diabetic patients and 158 non-diabetic patients who had received PKP and zoledronic acid treatment for OVCF were included and divided into group I (n=152, T2DM), and group II (n=158, non-T2DM). Oswestry disability index (ODI) and visual analogue scale (VAS) score was assessed. Operating time, bone mineral density (BMD), bone cement volume and therapeutic complications were recorded. MRI scan would be prescribed if a patient had back pain or suspected NVF.
Results: There was no significant difference in body mass index (BMI), BMD, bone cement volume, follow-up time, and fracture level between two groups (all p >0.05). All patients had improved VAS score, ODI and BMD at latest follow-up. However, VAS score and ODI were significantly lower in non-T2DM group (both p <0.001). There was no significant difference in incidences of NVF between two groups (11.2% vs. 8.9%, p=0.577). There were no severe therapeutic complications related with PKP or anti-osteoporosis treatment for OVCF.
Conclusion: Combination of PKP and zoledronic acid could effectively relieve pain and increase BMD in diabetic patients with OVCF and the risk of NVF was comparable with that in patients without diabetes.
Keywords: Osteoporosis, Vertebral fracture, Percutaneous kyphoplasty, Diabetes, Zoledronic acid
INTRODUCTION
Osteoporotic vertebral compression fracture (OVCF) was the most common fragile fracture and was associated with significantly lower quality of life and increased mortality in the elderly [1]. Compared with conservative treatment, percutaneous kyphoplasty (PKP) had the advantage of rapidly alleviating pain and stabilizing the vertebral body and enabling early mobilization [2]. However, no studies had addressed the clinical outcomes of PKP in patients with type 2 diabetes mellitus (T2DM) although numerous studies had reported that they had high risk of fracture [3].
The prevalence of new vertebral fracture (NVF) after PKP could reach up to 47.5% [4] and was a constant concern of spine surgeons [5]. There was a large amount of studies dedicated to decrease NVF incidence after PKP and found that anti-osteoporosis treatment was one of the most important elements [6] and anti-osteoporosis medications should be prescribed if there were no contraindications. However, when the patients complicated with T2DM, it would become much more complex. Patients with T2DM had lower bone quality and the medications for the treatment of T2DM might also have an impact on bone health [7]. More importantly, T2DM patients had increased risk of fall because of impaired physical function, nocturia and decreased vision due to retinopathy or cataract [8]. Thus,
T2DM patients had higher risk of OVCF even with a relatively better bone mineral density (BMD). In fact, the threshold of T-score was -2 for patients with T2DM [9]. Thus, it was reasonably to speculate that T2DM patients might had higher risk of NVF after PKP and it remained unknow whether anti-osteoporosis treatment could reduce NVF.
Regrettably, at present, because few studies, particularly no randomized clinical trials, had directly evaluated the efficacy of anti-osteoporosis treatment in diabetic patients, the anti-osteoporosis treatment in diabetic patients were empirical. Without strong evidence against, bisphosphonates remained the first choice and according to a recent systematic review, five studies (four studies with alendronate and one study with risedronate) had evaluated the efficacy of bisphosphonates in diabetic patients and the authors concluded that the presence of diabetes did not alter anti-osteoporotic treatment response regarding BMD increase and reduction of vertebral fracture [9].
Zoledronic acid belongs to the category of bisphosphonates and had been proven effective and safe [10]. In a large randomized trial involving women with low BMD or/and existing vertebral fractures, zoledronic acid resulted in significant lower rates of vertebral fractures (by 70%), hip fractures (by 41%) and non-vertebral fractures (by 25%) compared with placebo [11]. Besides, it has the advantage of high compliance because of its once-yearly administration [12]. Recently, a study by Zhang [13] demonstrated that there was no NVF in female patients who received zoledronic acid after PKP while 11.7% of the patients in the control group had NVF. However, no studies had evaluated its efficacy in T2DM patients or T2DM patients after PKP. The aim of the present study was to evaluated the efficacy of zoledronic acid in T2DM patients after PKP regarding the clinical outcomes, BMD increase and NVF reduction.
METHODS
This study was performed through retrospective analysis of the clinical and radiologic data. Approval from institutional review board was obtained.
Study population and treatment protocol
From January 2017 to December 2017, 152 diabetic patients who had received PKP and zoledronic acid were enrolled and 158 non-diabetic patients of similar age and BMD were included as the control group. The excluding criteria were: previous history of osteoporotic fracture, previous spinal surgery and other pathologic fractures (such as those related to malignancies or infections). Patients were divided into two groups: group I (n = 152, T2DM), group II (n = 158, non-T2DM). All participants received 1000mg calcium and 800IU vitamin D. Patients were allowed ambulation with a soft brace 6 hours after PKP. Before discharge, all patients received the first intravenous infusion of 5 mg zoledronic acid soluted in 100 ml saline and the second after 12 months and the third after 24 months. Patients received infusion of 750ml saline and took 0.3g ibuprofen orally before administration of zoledronic acid. Baseline data such as age, sex, body mass index (BMI, kg/m2), fracture level, the operating time, the bone cement volume and complications related with OVCF or PKP were recorded.
Assessments
Visual analog scale (VAS) and Oswestry Disability Index (ODI) were assessed at admission and at the latest follow-up. All patients had dual-energy X-ray absorptiometry (DEXA) scan of the lumbar and hip at admission but only had hip scan at the latest follow-up to measure the BMD. If a patient had acute back pain and suspected OVCF, a MR scan would be prescribed for verification. Complications associated with anti-osteoporosis medication were also recorded.
Statistical analysis
Data were presented as means ± SD. Continuous data such as age, follow-up time, BMI, operation time, bone cement volume, VAS score, ODI and BMD T score were analyzed by t-test. Categoric variables such as gender and NVF were analyzed by chi-square test. Statistical analysis was conducted using SPSS software (IBM SPSS Statistics, Version 24.0, Armonk, NY, USA). Statistical significance was set at p< 0.05.
RESULTS
Demographic characteristics
There was no significant difference in age (67.2 ± 7.6 vs. 66.8± 8.2 years), BMI (25.3 ± 3.1 vs. 24.9 ± 3.4 Kg/m2), bone cement volume (4.5± 0.7 vs. 4.6± 0.8 ml), hip baseline BMD T score (-3.2±0.5vs.-3.3±0.6,), follow-up time, operation time or distribution of fracture location between groups (Table 1).
Clinical outcomes
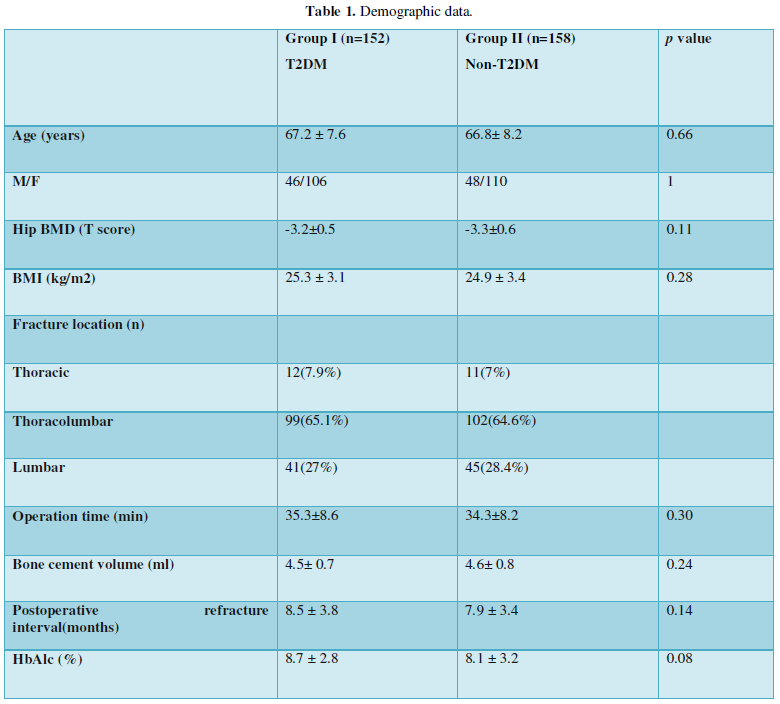
VAS score and ODI were similar between groups at admission. They were significantly lower in group II at the last follow-up (VAS score, 1.9 ± 0.7 vs. 1.4± 0.5, p<0.001; ODI 15.4±3.6% vs. 12.3±4.5%, p<0.001) and the improvement were-4.1± 0.8 vs. -4.5± 1.2, (p=0.045) (Figure 1a) and 40.4±3.3% vs. 45.2±3.8% (p<0.001) (Figure 1b) respectively.
BMD and NVF
Patients had similar BMD at admission and significantly better BMD at the final follow-up. Patients in group II had better improvement (0.49±0.07 VS. 0.56±0.11, p<0.001, Figure 1c) but the difference was minimal.
Seventeen NVFs (ten adjacent fracture and seven non-adjacent fracture) and fourteen NVFs (eight adjacent fracture and six non-adjacent fracture) occurred in group I and group II, respectively. There was no significant difference in terms of NVF (11.2% vs. 8.9%, p=0.577) between groups (Figure 1d). The interval between NVF and PKP was similar between groups (8.5 ± 3.8 VS. 7.9 ± 3.4 months, p=0.14, Table 1).
The gender differences
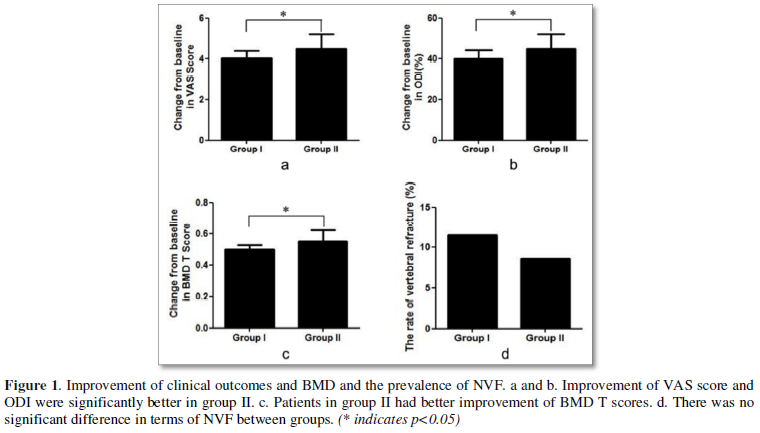
Female patients in Group II had significantly better improvement in VAS score (4.0±0.9 vs. 4.6±1.1, p=0.004, Figure 2a), ODI (40.8±2.8 VS. 45.5±3.6, p<0.001, Figure 2b) and BMD (0.51±0.07 vs. 0.56±0.12, p=0.032, Figure 2c) while there was no significant difference in the prevalence of NVF between groups (Figure 2d).
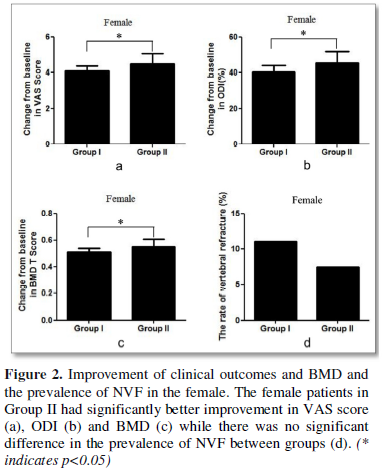
Male patients in Group II also had significantly better improvement in VAS score (3.9±0.7 VS. 4.5±0.9 p=0.039, Figure 3a), ODI (40.7±2.5 VS. 45.9±4.8, p<0.001, Figure 3b) and BMD T score (0.49±0.07 VS. 0.56±0.11, p=0.037, Figure 3c) and while there was no significant difference in the prevalence of NVF between two groups (Figure 3d).
Direct comparison demonstrated that female and male patients had similar improvement of VAS score, ODI and BMD and similar prevalence of NVF (Figures 4 & 5).
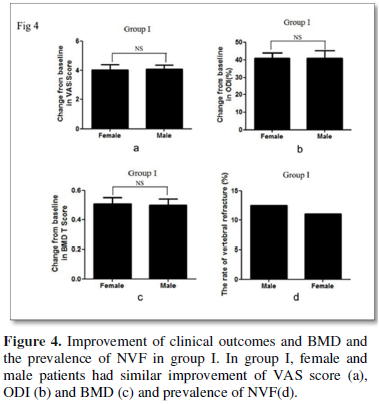
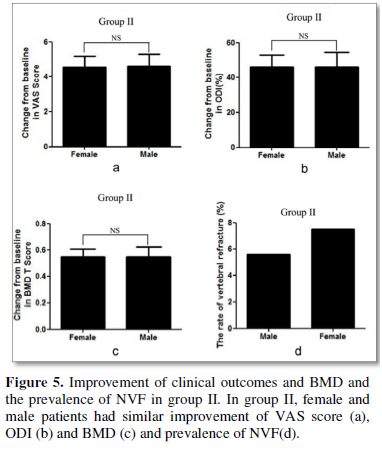






Adverse events
The common complications of zoledronic acid in the study included fever and muscle soreness, relieved by symptomatic treatment. There were no other severe complications.
DISCUSSION
Combination of PKP and zoledronic acid could significantly improve the clinical outcomes. Huang et al included 60 patients diagnosed with primary OVCF and treated with PKP and found that patients who received zoledronic acid had significantly lower back pain VAS score than patients in the control group [14]. Zhang [13] also observed similar advantages of zoledronic acid in patients after PKP. In the present study, both groups had significantly lower VAS score and ODI at the final follow-up. Patients without T2DM had statistically better clinical outcomes but the difference was minimal and might not have clinical significance, indicating T2DM patients could also expect good clinical outcomes.
According to a meta-analysis [15], the prevalence of NVF after vertebral argumentation was 16.4% but few studies had reported the prevalence of NVF in T2DM patients after PKP although numerous literatures had demonstrated that T2DM patients had higher risk of vertebral fracture. In the present study, the prevalence of NVF in T2DM patients after PKP was11.2%, while it was 8.9%in patients without T2DM, indicating that T2DM patients might not have higher risk of NVF after PKP.
Anti-osteoporosis treatment could reduce the risk of NVF after PKP and Zhang [13] demonstrated that zoledronic acid could reduce the prevalence of NVF to zero. In contrast, the prevalence of NVF after PKP remained high despite anti-osteoporosis treatment with zoledronic acid in the present study. Further analysis demonstrated that the mean interval between NVF and PKP was 8.5 months and 7.9 months respectively and the underlying explanation might be that the time to onset of anti-fracture efficacy of zoledronic acid was still not short enough to prevent NVF. In Boonen’s [4] study, 46.3% of the NVF occurred within 3 months in patients after PKP and 80.2% of the NVF occurred within 12 months. In another report, Boonen [16] demonstrated that patients with zoledronic acid had lower risk of vertebral fracture at 12 months and subsequent time-points but had similar risk of vertebral fracture at 6 months compared with patients with placebo. Thus, the spine surgeons should be aware that drugs with shorter time to onset of anti-fracture efficacy were warranted for patients after PKP because they had high risk of NVF shortly after PKP.
Despite evidence for increased risk of fracture in T2DM patients, little was known about the skeletal effects of anti-osteoporosis treatments in T2DM patients. As mentioned above, zoledronic acid had been proven effective and had good compliance but there were no studies focusing on the anti-osteoporotic treatment response of zoledronic acid in T2DM patients after PKP. The present study found that with the anti-osteoporosis treatment with zoledronic acid, although patients without T2DM had statistically better improvement of hip BMD, the difference was minimal and didn’t have clinical significance. This indicated that the presence of T2DM did not alter the anti-osteoporotic treatment response. Noticeably, despite BMD was one of the foremost determinants of bone strength and fracture risk, BMD might underestimate the fracture risk in T2DM patients. Cortical BMD was a major determinant of bone strength but BMD measured by DEXA could not distinguish cortical and trabecular cortical BMD. In fact, T2DM patients might have higher BMD but lower cortical BMD and high cortical porosity [17]. And it was demonstrated that T2DM patients experience fracture at a relatively high BMD [18]. For example, for a similar hip fracture risk, the BMD T-score in diabetic females and males was 0.59 and 0.38 higher than those in non-diabetic subjects [18]. What’s more, T2DM was an independent predictor of fracture, probably because of increased risk of falls [19].
Few studies had addressed gender difference of zoledronic acid. According to a recent meta-analysis, only four studies had investigated the effect of zoledronic acid in male patients [20], but the evidence available might be quite weak. Boonen [21] included 1199 men with primary or hypogonadism-associated osteoporosis. Compared with men receiving placebo, the prevalence of new morphometric vertebral fracture and moderate-to-severe vertebral fracture was lower in men who received zoledronic acid. However, in terms of clinical vertebral or nonvertebral fractures, the difference between zoledronic acid and placebo was not significant. In another study, Boonen [22] directly compare the outcomes between men and women and found that although the improvement of BMD was comparable with women, zoledronic acid did not decrease the clinical fracture compared with men who received placebo. Orwoll’s [23] study only evaluated BMD and bone turnover markers and the control group received alendronate rather than placebo and Kachnic [24] included male patients with advanced, non-metastatic prostate cancer receiving luteinizing hormone-releasing hormone agonist and radiotherapy, both of which might had not been designed well enough to elucidate the efficacy of zoledronic acid in male. Thus, there was limited evidence indicated its advantages in reducing clinical fracture in male. In the present study, we found that zoledronic acid could effectively improve BMD in male patients with and without T2DM and the improvement in BMD was comparable to female. The prevalence of NVF was also similar between male and female patients. Since zoledronic acid had been proven effective in female, the present study indicated zoledronic acid might be effective in male patients in terms of increasing BMD and preventing NVF.
As to safety, zoledronic had been proven safe. Acute phase response was common and the prevalence could reach up to 42.4%, including fever, musculoskeletal (pain and joint swelling), gastrointestinal (abdominal pain, vomiting, diarrhea), eye inflammation and general (including fatigue, nasopharyngitis, edema) [25]. In the present study, patients received infusion of 750 ml saline and 0.3g ibuprofen orally before zoledronic acid to prevent acute phase response. The adverse events observed in the present study included fever and muscle soreness and there were no severe adverse events. But the long-term safety of zoledronic acid in T2DM patients remained unknown in the present study as well as the literature available.
The present study had several limitations. First, this study was retrospectively designed and the sample size was small and had the potential of selection bias. Second, the study did not investigate the effect of zoledronic acid on bone turnover in T2DM patients. Third, the mean follow-up time was less than 2 years and the long-term efficacy of zoledronic acid in T2DM patients still remained unknown.
CONCLUSION
In this study, we firstly indicated that combination of PKP and zoledronic acid could effectively relieve pain and increase BMD in diabetic patients with OVCF and the risk of NVF was comparable with that in patients without diabetes. Prospective randomized controlled trials should be designed to further explore this largely unknown topic.
- Al-Sari UA, Tobias J, Clark E (2016) Health-related quality of life in older people with osteoporotic vertebral fractures: a systematic review and meta-analysis. Osteoporos Int 27: 2891-2
- Zhu R-S, Kan S-L, Hu W, Ning G-Z, Chen L-X, et al. (2019) Which is the best treatment of osteoporotic vertebral compression fractures: balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos Int 30: 287-298.
- Koromani F, Oei L, Shevroja E, Rivadeneira F, Trajanoska K, et al. (2020) Vertebral Fractures in Individuals with Type 2 Diabetes: More Than Skeletal Complications Alone. Diabetes Care 2020; 43:137-144.
- Boonen S, Van Meirhaeghe J, Wardlaw D, Bastian L, Cummings SR, et al. (2011) Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 26: 1627-1637.
- Zhang H, Xu C, Zhang T, Gao Z, Zhang T (2017) Does Percutaneous Vertebroplasty or Balloon Kyphoplasty for Osteoporotic Vertebral Compression Fractures Increase the Incidence of New Vertebral Fractures? A Meta-Analysis. Pain Physician 20: E13-E28.
- Lee DG, Park CK, Hwang JH, Park CJ, Lee DC (2015) Analysis of Risk Factors Causing New Symptomatic Vertebral Compression Fractures After Percutaneous Vertebroplasty for Painful Osteoporotic Vertebral Compression Fractures: A 4-year Follow-up. J Spinal Disord Tech 28: E578-E583.
- Lin DPL, Dass CR (2018) Weak bones in diabetes mellitus - an update on pharmaceutical treatment options. J Pharm Pharmacol 70: 1-17.
- Kanazawa I, Takeno A, Sugimoto T, Tanaka K-I, Yamane Y (2018) Osteoporosis and vertebral fracture are associated with deterioration of activities of daily living and quality of life in patients with type 2 diabetes mellitus. J Bone Miner Metab 37: 503-511.
- Ferrari SL, Abrahamsen B, Napoli N, Akesson K, Bone and Diabetes Working Group of IOF, et al. (2018) Diagnosis and management of bone fragility in diabetes: An emerging challenge. Osteoporos Int 29: 2585-25
- MacLean C, Newberry S, Grossman J, Maglione M, McMahon M, et al. (2008) Systematic review: Comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med 148: 197-213.
- Black DM, Delmas PD, Eastell R, Reid IR, HORIZON Pivotal Fracture Trial, et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809-1822.
- Moriwaki K, Mouri M, Hagino H (2017) Cost-effectiveness analysis of once-yearly injection of zoledronic acid for the treatment of osteoporosis in Japan. Osteoporos Int 28: 1939-1950.
- Zhang J, Zhang T, Zhao D, Xu X, Cai Q (2019) Zoledronic acid combined with percutaneous kyphoplasty in the treatment of osteoporotic compression fracture in a single T12 or L1 vertebral body in postmenopausal women. Osteoporos Int 30: 1475-1480.
- Huang ZF, Xiao SX, Liu K, Xiong W (2019) Effectiveness Analysis of Percutaneous Kyphoplasty Combined with Zoledronic Acid in Treatment of Primary Osteoporotic Vertebral Compression Fractures. Pain Physician 22: 63-68.
- Lou S, Shi X, Wang Y, Zhang X, Lyu H, et al. (2019) Percutaneous vertebroplasty versus non-operative treatment for osteoporotic vertebral compression fractures: A meta-analysis of randomized controlled trials. Osteoporos Int 30: 2369-2380.
- Boonen S, Eastell R, Black DM, Su G, Mesenbrink P, et al. (2012) Time to onset of antifracture efficacy and year-by-year persistence of effect of zoledronic acid in women with osteoporosis. J Bone Miner Res 27: 1487-1493.
- Ho-Pham LT, Chau PMN, Nguyen TV, Do AT, Nguyen HC (2018) Type 2 diabetes is associated with higher trabecular bone density but lower cortical bone density: The Vietnam Osteoporosis Study. Osteoporos Int 29: 2059-2067.
- Tao B, Liu J-M, Ning G, Zhao H-Y, Sun L-H, et al. (2008) Differences between measurements of bone mineral densities by quantitative ultrasound and dual-energy X-ray absorptiometry in type 2 diabetic postmenopausal women. J Clin Endocrinol Metab 93: 1670-1675.
- Yamamoto M, Yamaguchi T, Sugimoto T, Yamauchi M, Kaji H (2009) Diabetic patients have an increased risk of vertebral fractures independent of BMD or diabetic complications. J Bone Miner Res 24: 702-709.
- Zeng L-F, Pan B-Q, Liu J, Liang G-H, Luo M-H, et al. (2019) Does Routine Anti-Osteoporosis Medication Lower the Risk of Fractures in Male Subjects? An Updated Systematic Review with Meta-Analysis of Clinical Trials. Front Pharmacol 10: 882.
- Boonen S, Reginster J-Y, Orwoll E, Kaufman J-M, Lippuner M, et al. (2012) Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med 367: 1714-1723.
- Boonen S, Orwoll E, Magaziner J, Colón-Emeric CS, HORIZON Recurrent Fracture Trial, et al. (2011) Once-yearly zoledronic acid in older men compared with women with recent hip fracture. J Am Geriatr Soc 59: 2084-2090.
- Orwoll ES, Miller PD, Weinstein RS, Adachi JD, Brown J, et al. (2010) Efficacy and safety of a once-yearly IV infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: A randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res 25: 2239-2250.
- Kachnic LA, Pugh SL, Lawton CA, Tai P, Smith M, et al. (2013) Randomized phase III trial to evaluate zoledronic acid for prevention of osteoporosis and Associated fractures in prostate cancer patients. Prostate Cancer Prostatic Dis 16: 382-386.
- Reid IR, Gamble GD, Mesenbrink P, Lakatos P, Black DM (2010) Characterization of and Risk Factors for the Acute-Phase Response after Zoledronic Acid. J Clin Endocrinol Metab 95: 4380-4387.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Renal Transplantation Science (ISSN:2640-0847)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Oncology Clinics and Research (ISSN: 2643-055X)







