Research Article
Fire Investigation in an Industrial Grade Nitrocellulose Plant
6235
Views & Citations5235
Likes & Shares
An incident of fire was reported in an industrial grade nitrocellulose manufacturing unit. Forensic investigation was carried out to find the cause of fire. The scene of fire was thoroughly examined and searched for the evidence. Partially burnt samples of debris and burnt electrical wires were collected from the scene of fire. The GC-MS analysis of the debris revealed the presence of hydrocarbons belonging to fire accelerants. A study of CCTV footages revealed the origin and time of fire. Basing on the findings and the information gathered during field visit, it was opined that the incident of fire was caused due to human intervention.
Keywords: Nitrocellulose, Industrial grade, Isopropyl alcohol, Butanol, GC-MS
INTRODUCTION
Nitrocellulose consists of two grades viz., Propellant grade containing more than 12.5 % nitrogen used for production of smokeless gun powders and Industrial grade containing less than 12.5% nitrogen and employed as a film-forming agent in solvent-based paints, protective coatings, fingernail polishes etc. Nitrocellulose (Figure 1) is a derivative of cellulose and is manufactured from purified cellulose either in the form of cotton linters or wood pulp. The manufacturing process involves nitration of purified cellulose with nitric acid and Sulphuric acid under controlled conditions followed by stabilization and washing.
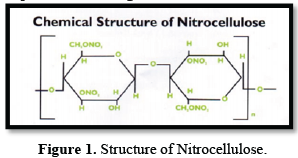

The fire affected plant manufactures industrial grade nitrocellulose by removing water from wet nitrocellulose by centrifuging and adding a solvent such as isopropyl alcohol/butanol. Wet nitrocellulose with nitrogen content in the range of 10.7% to 12.2% is taken as raw material.
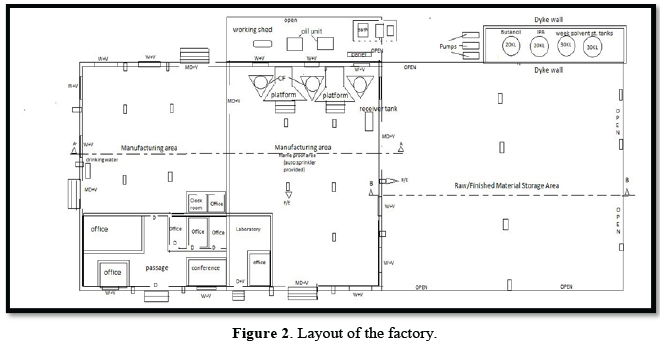
Pallets carrying these drums are transferred to the designated storage area. Tankers carrying solvents- isopropyl alcohol/butanol are unloaded in MS storage tanks. During centrifuging process, the industrial wet nitrocellulose is manually taken in open top HDPE drums and using SS bowls, the centrifuges are charged. The centrifuge machine is operated between 500-900rpm for the desired duration. After obtaining the desired moisture content of 2-3%, isopropyl alcohol/butanol is mixed vigorously by using wooden shovel manually. Once the industrial damped nitrocellulose becomes ready, it is packed into corrugated/MS drums. According to security guard, he closed and locked the plant at 10:00pm as usual after the night shift. In the early hours between 03:00 to 03:30 am on the fateful day, he heard some crackling noise and immediately got alerted and rushed to investigate when he noticed huge flames and smoke emanating from the manufacturing area of the plant. He informed the fire brigade which reached in the next 25 min and brought the flames under control by 9:00 am. There was no explosion and there were no casualties in the incident. The factory (with RCC roofing) consisted of two halls, one on the northeastern side (13mx16m) meant for raw material storage area and the adjacent hall (15mx22m) called manufacturing area where three centrifuges were arranged adjacent to the southeastern wall. Behind the manufacturing area of the RCC building and the southeastern compound wall, working shed (15.5mx4m), with GI sheets roofing was located and contained one air compressor unit, two oil units & electrical panel board. Dyke wall of height 0.60 m was present behind the southwestern area where four tanks (one for Butanol 20 KL, one for I.P.A. 20KL and two solvent storage tanks each of capacity 30KL) were located. On the southwestern side of the manufacturing area finished material storage area (22mx25m) with GI Sheeted roof was located as shown in Figure 2 below.

OBSERVATIONS & FINDINGS
In the raw material storage area, finished products (solvent nitrocellulose) in different sizes of drums were stored in addition to raw material (wet nitrocellulose). All the material stored in the storage area was found to be partially burnt (Figure 3).

The manufacturing area contained three centrifuges (Figure 4) on the southeastern side adjacent to the wall which were superficially affected by fire. 200 drums kept in the manufacturing area were found to be partially burnt.


The finished product storage area (Figure 5) (adjoining the manufacturing area) was found to be partially affected by fire. The solvent tanks in the finished product storage area were found to be totally intact and were not affected by fire.


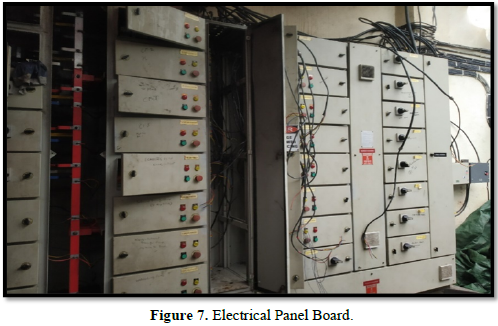
The working shed located behind the building of the manufacturing and raw material storage area was found to be superficially affected by fire. Even though the roof was burnt, the drums and panel boards located there were not affected by fire (Figure 6).


The electrical panel located in the working shed was not affected by fire (Figure 7).

RESULTS & DISCUSSION
Five partially burnt electrical wires and five samples of partially burnt debris were collected from different locations inside the manufacturing area as mentioned.
- Electric wires collected from the remaining burnt wires on the southwestern side of the wall between the manufacturing area and finished material area.
- Electric wires collected from the remaining burnt wires on the southwestern side of the wall between the manufacturing area and finished material area.
- Electric wires collected from the south-eastern wall behind the centrifuges located in the manufacturing area.
- Electric wires collected from the south-eastern wall behind the centrifuges located in the manufacturing area.
- Electric wire sample collected on the north-eastern side of the wall in the raw material storage area.
- Partially burnt debris sample collected adjacent to main door besides drinking water container.
- Partially burnt debris sample collected on the north-eastern side of the wall in the raw material storage area.
- Partially burnt debris sample collected underneath the southwest main door in the manufacturing area.
- Partially burnt debris sample collected underneath the north-western side window of manufacturing area.
- Partially burnt debris sample collected from the laboratory.
The stereomicroscopic examination of the burnt wires did not show any symptoms of short circuit such as Beading (one of the images is shown in (Figure 8).

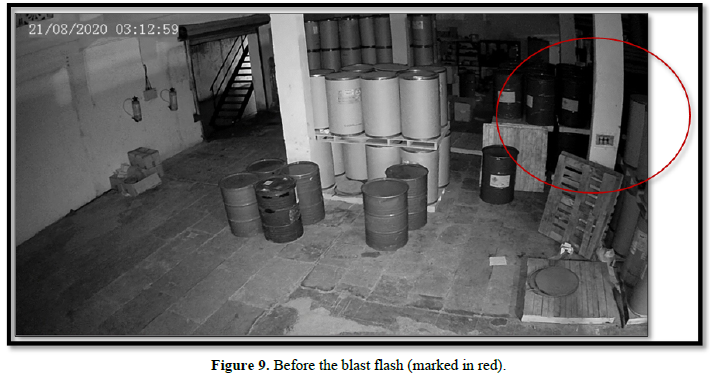




The CCTV footages of Cam 6 located near the water tank in the compound wall besides the working area (and behind the raw material storage area) also showed a flash of light at 03:13:01. From the above, it is concluded that the fire originated at 03:13:01 with a burst of flame in the manufacturing area. This could happen only due to sudden ignition of solvents Isopropyl Alcohol, Butanol and nitrocellulose that were used in the manufacturing process. These substances even though flammable require external ignition to catch fire. At the time of fire incident, there was no manufacturing activity in the factory and no high-power drawing machinery/equipment was being used. The transformer and its circuitry were found to be in normal working order and no symptoms of short circuit were noticed in the electrical wires collected in and around the switch board of the manufacturing area such as beading and hence fire due to short circuit or any other electrical related causes was ruled out. As the plant was closed, fire caused due to friction due to some worker inadvertently dragging the drums on the concrete floor was also ruled out. As there was tight security, the workers/employees bringing cigarette/ match boxes into the premises and consequent fire due to negligence was ruled out. The results of GC-MS analysis of the fire debris samples collected at four out of five locations in the plant showed the presence of hydrocarbons belonging to fire accelerants such as diesel/kerosene. As these hydrocarbons cannot be found in the combustion products of the solvents used for manufacturing purposes, their presence in the debris collected indicates an external ingress of diesel/kerosene. Further, the detection of hydrocarbons at four different locations inside the factory also indicates multiple origins of fire. However, these could not be captured in the CCTV footages as no camera was focused on these areas.
CONCLUSION
Based on a thorough and in-depth inspection of the fire incident followed by search, identification, collection, analysis of physical and oral evidence and applying fire dynamics, it is concluded that, the fire was due to human intervention in deliberately setting fire by using fire accelerants.
- Abel RJ, Zadora G, Sandercock PML, Harynuk JJ (2018) Modern instrumental limits of identification of ignitable liquids in forensic fire debris analysis. Separations 5(4): 58.
- Twibell JD, Home JM, Smalldon KW (1982) A comparison of the relative sensitivities of the adsorption wire and other methods for the detection of accelerant residues in fire debris. J For Sci Soc 22(2): 155-159.
- Thatcher PJ (1986) The scientific investigation of fire causes. In Forensic Science Progress, Springer, Berlin, Heidelberg pp: 117-151.
- Choodum A, Daeid NN (2011) Development and validation of an analytical method for hydrocarbon residues using gas chromatography-mass spectrometry. Anal Methods 3(5): 1136-1142.
- Nowicki J (1990) An accelerant classification scheme based on analysis by gas chromatography/mass spectrometry (GC-MS). J For Sci 35(5): 1064-1086.
- ASTM (1994) ASTM E 16 Standard Guide for Ignitable Liquid Residues in Extracts from Fire Debris Samples by Gas Chromatography-Mass Spectrometry. ASTM, Philadelphia.
- Gilbert MW (1998) The use of individual extracted ion profiles versus summed extracted ion profiles in fire debris analysis. J For Sci 43(4): 871-876.
- Kirk’s Fire Investigation Gas Chromatography/ Mass Spectrometry (GC/MS) (2007) 6th edn pp: 530-547.
- Murthy TSN, Kumar P, Mohan GE (2020) Intentionally is setting fire to JCB excavator for fraudulent insurance claim. Royal J For Sci 8(1): 12-15.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Alcoholism Clinical Research
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Ophthalmology Clinics and Research (ISSN:2638-115X)












