Case Report
Forensic Detction and Identification of Tramadol: A Case Report
3619
Views & Citations2619
Likes & Shares
Tramadol, a commonly prescribed opioid analgesic, is considered to have a low abuse potential and devoid of side effects like drug dependence. A few fatalities due to tramadol overdose, either intentional or accidental, have been reported in the literature. We report a case of a 17-year-old female who died with tramadol overdose. Some patients might exhibit a certain degree of tolerance to the drug after prolonged prior exposure to the medication, and this tolerance might extend beyond the therapeutic range. This also emphasizes the need for physicians to be more cautious while prescribing tramadol to their patients.
Keywords: Isolated, Opioid, Poisoning, Tolerance, Tramadol
INTRODUCTION
Tramadol is a synthetic opioid analgesic centrally acting agent. It exerts its analgesic effect by inhibiting the re-uptake of nor epinephrine and serotonin and also by weak opioid receptor agonism, mechanisms that are due to tramadol and its active metabolite o-desmethyltramadol. Tramadol also consists of 2 enantiomers, (+) – tramadol, which preferentially inhibits serotonin uptake and (-)- tramadol, which is the potent inhibitor of nonepinephrine reuptake [1-4].
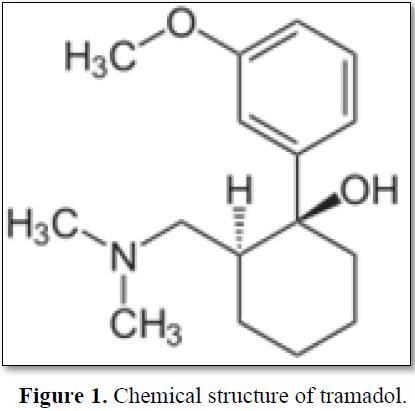
It is used for the treatment of moderate to severe pain. Tramadol dosage should be adjusted according to the severity of pain. The total daily dose should not exceed 400mg with adult therapeutic blood levels of 0.1–0.8 mg/L [5]. We report a case of an adult female with tramadol overdose having a lethal blood concentration and who died with serious adverse effects. Tramadol hydrochloride is a white, bitter, crystalline and odorless powder. It is readily soluble in water and ethanol, sold in market with proprietary names Dromadol, Tramake, Zydol etc. The molecular weight of tramadol hydrochloride is 299.8, C16H25NO2.HCl (±) cis-2- [(dimethyl amino) methyl]-1-(3methoxy phenyl) cyclohexanol hydrochloride. Its structural formula is given in Figure 1.
DISPOSITION IN THE BODY
Tramadol is rapidly and almost completely absorbed after oral or parenteral administration. The presence of food does not significantly affect the rate or extent of absorption. Tramadol is extensively metabolized with metabolic reactions N- and o-demethylation and conjugation with glucuronic acid and sulfate. The major metabolites formed are o-monodesmethyltramadol, N, O-didesmethyltramadol and their conjugates, and N-monodesmethyltramadol. o-Monodesmethyl tramadol is an active metabolite and has a greater analgesic activity than the parent drug. About 90% of an oral dose is excreted in the urine in 3 days, about 30% of the dose as unchanged drug and the rest as metabolites. The remainder of the dose is eliminated in the faeces. The rate of excretion is reduced in patients with renal impairment. The drug is widely distributed throughout the body and detected in breast milk.

CASE HISTORY
A 17-year-old female was brought dead by the relatives to the hospital at 8.0 am. She was found by her parents shrieking in her room around 7:00am and they found the patient confused and unable to recognize them. As per attendant she had a background history of suffering from headache intermittently for the past 3 years. She was prescribed tramadol. She had been taking tablets of tramadol per day as needed over the last year. She had a persistent headache that night which was unrelieved despite taking a regular dose of tramadol as prescribed. She only remembered that she took more than the usual number of tablets of tramadol but was unable to quantify the exact number. The exact time of consumption of tablets was unknown.
EXPERIMENTAL
Standard reagents, bismuth subnitrate, acetic acid, potassium iodide used were AR grade. Tramadol pure, doubly distilled water was used throughout the study.
Preparation of standard stock solutions
Standard stock solution of concentration 1000 μg/mL for tramadol were prepared using methanol. From the standard stock solution, the mixed standard solutions were prepared using methanol to contain 100 μg/mL of tramadol. The stock solution was stored at 2-8°C protected from light.
Preparation of chromogenic reagent
Solutin (a) 2 g of bismuth subnitrate and 25 mL of acetic acid together mixed in 100 mL of water; solution (b) 40 g of potassium iodide dissolved in 100 mL of water. Add 10 mL of solution (a) and 10 mL of solution (b) in 20 mL of acetic acid and make up in 100 mL of water.
Samples: Ttramadol tablets and autopsy tissue.
EXTRACTION OF TRAMADOL FROM AUTOPSY
TISSUE AND CLEANUP OF EXTRACTS
In a portion of about 100 g of autopsy tissues (stomach, intestine, lung, liver, spleen and kidney) containing the tramadol drug, 10 g Ammonium sulphate was added and minced. Then biological sample was made alkaline with the help of ammonia and extracted with methanol. The filtrate was evaporated. The extracts were subjected to clean up by passing through the mixture of silica gel G and activated charcoal filled column having glass wool at the bottom. Finally, the collected filtrate was evaporated over hot water bath and used for identification of tramadol.
Thin layer chromatographic analysis
Aliquots of standard tramadol and extract obtained were spotted on to the plate, which was developed with ethyl acetate methanol ammonium hydroxide in the ratio of (85: 10: 5) (v/v/v); it gave spot of tramadol at Rf value 0.76, in a pre-saturated TLC chamber, to a height of 10 cm. The plate was removed from the chamber dried in air and sprayed with chromogenic reagent, which forms orange-colored spots against white background. The RF value of tramadol can be compared with the obtained spots of extract.
TLC method optimization and chromatographic conditions
The TLC procedure was optimized for estimation of tramadol. The standard stock solution 100 μg/mL of tramadol) were taken and 10 μL samples were spotted on to TLC plates and run in different solvent systems. Initially, toluene, acetone and methanol were tried in different ratios, but perfect spots were not obtained. Hence, ammonia was tried along with above mobile phase. Finally, for effective separation of tramadol, the mobile phase containing a mixture of ethyl acetate methanol ammonium hydroxide in the ratio of (85: 10: 5) (v/v/v) Toluene: Acetone: Methanol: Ammonia (7: 1.5: 1: 0.1 v/v/v/v) was found to be optimum. The above mobile phase improved the spot shape and gave suitable Rf value for tramadol. In order to reduce the neck less effect TLC chamber was saturated for 30 min. The plates were developed and then dried in hot air, which takes approximately 20 min for complete development of the TLC plate.
Gas chromatograph mass spectrometer
GC-MS studies were performed on Agilent technologies 5973 inert model mass selective detector using Column HPSMS 0.25 mm id 30 m length, 0.25m film thickness, 30mx250mmx0.25mm nominal with aux temp 280°C, intel temp 250° MS quadrupole 150 ion source 230, column flow 1.0 ml/min He as carrier gas, split mode 20:1 programming 100°C 2 min hold 20°C/min ramp up to 280°C, 500 volt total 16 min run.
RESULTS AND DISCUSSION
Tramadol is a commonly used opioid for the management of moderate to severe pain. Therapeutic dosage is 50-100 mg every 4-6 h, to a maximum recommended dose of 400 mg/day. It is rapidly and completely absorbed with a peak blood concentration within 2 h. Tramadol can induce physical and psychological dependence. Subjects with a history of substance abuse are at higher risk. But the dependence is not limited to those patients with a prior history of opioid dependence or substance abuse. This has led to increasing incidence of tramadol related fatalities in case reports and post-marketing surveillance reports. When taken in overdose it is known to be associated with significant morbidity and mortality. Moreover, fatal intoxications with tramadol may also occur unintentionally. The most common symptoms of tramadol overdose are central nervous system (CNS) depression, nausea and vomiting, tachycardia and seizures. Higher doses can be associated with classic opioid toxicity features of coma, respiratory depression and cardiovascular collapse. Tramadol overdose has been one of the most frequent causes of drug poisoning in the recent years, especially in young adult males with a history of substance abuse and mental disorders. Tramadol is increasingly being prescribed as an analgesic in India. Although tramadol toxicity may occur following intentional or accidental ingestion of the drug, fatality may not occur in all the cases. There might be a possibility of individual variation and some patients might exhibit a certain degree of tolerance to the drug after chronic ingestion or prolonged prior exposure to the medication, and this tolerance could extend beyond the therapeutic range, as happened in this case the toxicological analysis of extract shown a tramadol level of 4 mg/L, against the therapeutic range of 0.1-0.84 mg/L.
Tramadol can be successfully detected on TLC plates with good sensitivity.
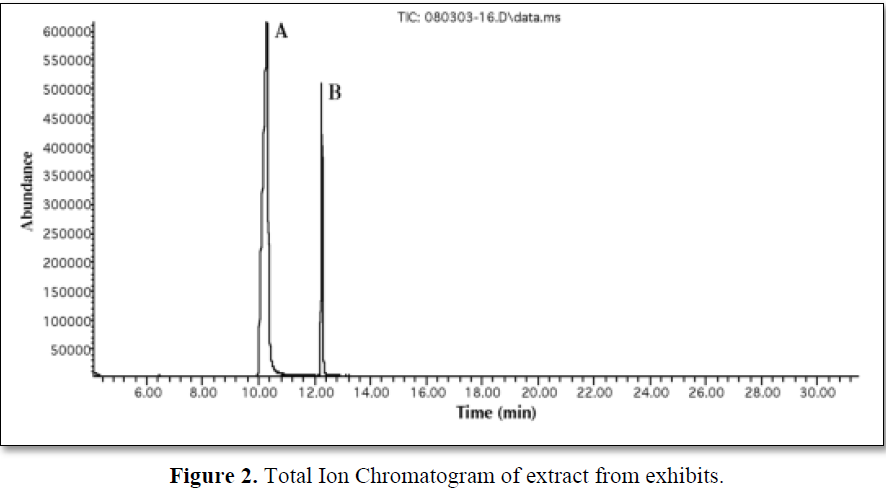
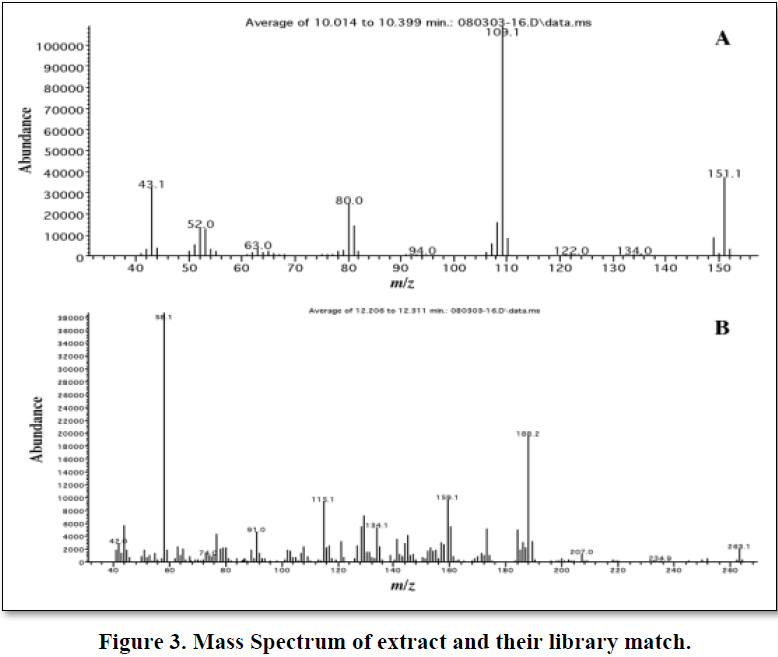
The extract was injected into the GC-MS apparatus, and a total ion chromatogram and a full scan mass spectrum were obtained. Figure 2 shows the TIC and Figure 3 shows the mass spectrum of tramadol with their library match, in this work; we attempted to detect tramadol using selected ion monitoring. The detection limit was determined by analyzing samples at various concentrations with this method, and it was found that 0.01 mg/ml of residual tramadol in a sample can be detected using SIM. Figure 2 shows the total ion chromatogram at equal sensitivity of extracts from blank samples. The peak of tramadol can be clearly identified; thus, the detection limit was determined to be at least 0.01 mg/ml in sample. However, many background ions appeared on the full scan mass spectrum of this peak.

CONCLUSION
In summary, nowadays tramadol is a common drug for pain control, it is important for physicians to be aware of its potentially lethal side effects, particularly if consumed or prescribed in inappropriately large doses, or when it interacts with other medications. It develops physical and psychological dependence. Therefore, physicians have to be more cautious while prescribing tramadol to their patients. TLC is rapidly becoming a routine analytical technique due to its advantages of low operating costs, high sample throughput, and the need for minimum sample preparation. The reported TLC technique is precise, specific, and accurate and cost effective. The method is simple, fast and reliable with no interference from common drugs. The method developed and the analysis of tramdol in tissues could prove that the tramadol was taken by deceased and the subsequent reaction had caused the death.
ACKNOWLEDGEMENT
Author is thankful to Director Forensic Science Laboratory, Sagar, in charge Joint Director R.F.S.L. Gwalior for providing necessary facilities and Director, D.R.D.E. Gwalior for GC-MS spectral study.

- Moore KA, Cina SJ, Jones R, Selby DM, Levine B, et al. (1999) Tissue distribution of tramadol and metabolites in an overdose fatality. Am J Forensic Med Pathol 20: 98-100.
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, et al. (1992) Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ‘atypical’ opioid analgesic. J Pharmacol Exp Ther 260: 275-285.
- Clarot F, Goulle JP, Vaz E, Proust B (2003) Fatal overdoses of tramadol: Is benzodiazepine a risk factor of lethality? Forensic Sci Int 134: 57-61.
- Tjäderborn M, Jönsson A K., Hägg S, Ahlner J (2007) Fatal unintentional intoxications with tramadol during 1995-2005. Forensic Sci Int 173: 107-111.
- Chandrasekaran D, DeSilva P, Dhatariya K (2007) An uncommon presentation of a common drug overdose - The dangers of under estimating tramadol. J Med Sci Res 1: 59-61.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Clinical Case Studies and Reports (ISSN:2641-5771)
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Oncology Clinics and Research (ISSN: 2643-055X)
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Journal of Immunology Research and Therapy (ISSN:2472-727X)
- International Journal of AIDS (ISSN: 2644-3023)
- Journal of Alcoholism Clinical Research





