3474
Views & Citations2474
Likes & Shares
Although the notion that cancerous cells cannot revert to their original and healthy states is widely accepted, this idea might no longer be valid. My team reported a noteworthy function of hsa-miR-520d-5p (520d-5p), such as the induction of demethylation, CD105 upregulation, and P53 upregulation, via stemness mediated-mechanism in cancer cells as well as normal cells. Also, we described presumably key roles for p53, AIDA, Nanog, and the 520d-5p target genes, including ELAVL2, TEAD1, SBF2, PUM2, GATAD2B, and SEH1L, in a benign conversion process, in which human hepatoma cells were converted to healthy/benign phenotypes via stemness induction, resulting in a reversal of malignancy in vivo. We showed that this small molecule induced spheroid formation with CD105 positivity in fibroblasts and we also confirmed the therapeutic effect of inducing 520d-5p in lethally UV-damaged fibroblasts. Subsequently, through next-generation sequencing analysis in hepatoma cells and human iPSC-derivative cells in pre-mesenchymal stem cell status, we confirmed the restoration of a single nucleotide mutation in diverse genes involved in gene expression by inducing 520d-5p and confirmed the alterations in metabolites using both genome-wide and individual gene function approaches. 520d-5p induced a shift toward a wild type or non-malignant phenotype, which was regulated by nucleotide mutations in both hepatoma cells (HLF) and human induced pluripotent stem cells (hiPSCs). Furthermore, 520d-5p reduced mutation levels in both the whole genome and genomic fragment assemblies, although the genomic mutations in cancer cells could not be repaired in most contexts. These findings suggest that the development of novel applications of 520d-5p would enhance cancer or antiaging research and contribute to the qualitative improvement of hiPSCs or their derivatives, including human mesenchymal stem cells (hMSCs), used in regenerative medicine.
Keywords: Mutation, iPSC, Progenitor hMSC, Hsa-miR-520d-5p, Genomic conversion
Abbreviations: 520d-5p: hsa-miR-520d-5p; hiPSCs: Human Induced Pluripotent Stem Cells; hMSCs: Human Mesenchymal Stem Cells; miRNA: microRNAs; NGS: Next-Generation SequencingINTRODUCTION
Human microRNAs (miRNAs) have diverse biological functions, and play a role in nearly every biological process. We have scattered more than 7000 reports on miRNA published since 2010, and we observed a rapid increase in the number of reports published in the past 2 to 3 years on the reprogramming effects of miRNAs. We were the first in the world to report that miR-520d-5p (520d-5p) caused undifferentiated cancer cells to adopt benign or healthy status in vivo via demethylation and P53 up regulation [1]. My team further found that 520d-5p causes normal cells (fibroblasts) to extend their lifespan and mesenchymal stem cell-like status with CD105 positivity [2]. DNA that has undergone fragmentation can be restored to its original status such that damaged cells can survive [3]. Although we hypothesized that ectopic 520d-5p expression reduced mutations through the synergistic modulation of methylation-related enzymatic expression, next-generation sequencing (NGS) analysis partly clarified the mechanism underlying the phenomena [4]. Mutations in the genome could be converted to the wild type, suggesting the feasibility of applying the mechanism to anti-cancer therapy, anti-aging therapy including brain aging or blood vessel aging, and regenerative medicine. Here, we present a summary of recent findings that deserve attention with respect to miRNAs, and we review the possible functions of miRNA and directions for the application of the associated science.
REVIEW
Nowadays researchers in the world are examining the projects covering new approaches for small molecule drug discovery and optimization. It has been reported that miRNAs are associated with the biology of cancer, anti-cancer activities, metastasis, and the improvement of the tumor environment. We hereby introduce miRNAs which play major parts in the application for anti-cancer, anti-aging, reprogramming, and the conversion to stemness status, including our projects, among the many articles published.
CANCER
It has been reported that miRNAs reprogram fibroblasts to become cancer associated fibroblasts (CAFs); therefore, the mechanisms underlying carcinogenesis caused by oncomiR and the anti-cancer effect of suppressor miR have been investigated [5]. Androgen signalling is associated with the function of miRNA, including expressive heterogeneity in prostate cancer progression [6,7]. In colorectal cancer, there is evidence to suggest that miRNAs contribute to several aspects of tumorigenesis and that inhibition of highly expressed miRNAs or their replacement by miRNAs with reduced expression could become treatment strategies [8]. Metabolome profiles have shown that cancer-specific enzymatic changes and miRNAs regulate the action of methionine aminopeptidase or N-myristoyltransferase [9]. miRNAs play a crucial role in the expression of tumor suppressor genes. TP63, a member of the TP53 family, is also involved in these functions and is both physically and functionally connected with STAT3, which is an important regulator of both healthy stem cells and cancer stem cells [10]. miRNAs in particular are integral components of the TP53 network, regulating multiple p53-controlled biological processes to modulate the differentiation and self-renewal potential of stem cells [11]. Loss of X Inactive Specific Transcript (XIST) also augmented the secretion of exosomal miRNA-503, which triggered M1/M2 polarization of microglia. This M1/M2 conversion upregulated immune suppressive cytokines in microglia, resulting in suppressed T-cell proliferation [12]. miRNAs seem to be closely associated with the TP53 and SIRT families. We previously reported that 520d-5p up regulates the expression of TP53 and SIRT 1, inducing an anti-cancer effect and converting cells to a nonmalignant status [13-15] (Figures 1 and 2).
Metabolome analysis (heat map) revealed metabolomic changes from cancer status to non-cancer status 7 days later than transfection with 520d-5p. On Day 5, almost all the metabolites were shut down and reset. Transfectants (circled in red) showed an entirely different profile compared with that of parental cancer cells (circled in blue) in metabolites. 5D and 7D: 5 and 7 days after transfection. R1 and R2: transfectants sorted using pluripotent markers (they cannot give rise to any malignant tumors).
ANTI-AGING AND REPROGRAMMING
There have been numerous reports of the reprogramming of fibroblasts by miRNA. Several pro- and anti-aging factors have been identified and many miRNAs (including miR-17, miR-125b and miR-181 family members) have been linked with the age-dependent dysregulation associated with various physiological processes [16]. Natural products, or nutraceuticals, can elicit anti‐aging, anti‐cancer, and other health‐enhancing effects. A key target of the effects of natural products is the regulation of miRNA expression, which can result in cell death or help prevents aging and many diseases. The effects were involved in the expression of TP53/miR-34a/SIRT-1 axis induced by natural products (resveratrol, curcumin, etc). For example, the down regulation of miR-34, 145, and 200c can inhibit an aging or apoptosis [17]. Recently, nicotinamide mononucleotide (NMN) was shown to potentially regulate miRNA-regulated anti-aging mechanisms, and NAD+ booster treatments and sirtuin activators could be harnessed for the development of new pharmacological approaches for the prevention and treatment of age-related vascular diseases [18]. Many miRNAs are linked with the age-dependent dysregulations of various physiological processes, including stem cell aging. All miR-17, miR-125b, and miR-181 family members are down regulated in various old tissue stem cells (TSCs), and their down regulation suppresses cytogenesis, proliferation, and secretion of homeostatic factors, while promoting inflammation and tumorigenesis [16]. The knockdown of miR-124 results in cephalic regeneration phenotypes, such as in the brain and visual systems. Furthermore, the concentration of reactive oxygen species (ROS) can modulate TGF-β activation, which in turn down regulates two types of miRNAs (miR-200 and miR-302). Surprisingly, these two miRNAs maintain pluripotency, while they are down regulated during the acquirement of a specific cellular phenotype. Hypoxia can deeply influence stem cell behavior by inducing the appearance of specific phenotypes as well as the direct reprogramming of somatic cells [19].
Exosomes produced by local fibroblasts in the muscles of individuals with Duchenne muscular dystrophy (DMD) can induce the phenotypic conversion of healthy fibroblasts to myofibroblasts, thereby increasing the fibrotic response [20]. The conversion of myeloid to fibroblast-like cells is impaired in wounds of individuals with diabetes. During cross-talk between keratinocytes and myeloid cells, miR-21 packaged in extracellular vesicles (EV) is required for cell conversion [21]. A combination of miRNAs 1, 133, 208, and 499 is capable of inducing direct cellular reprogramming of fibroblasts to cardiomyocyte-like cells in vitro [22]. The restoration of tissue homeostasis by controlling stem cell aging is a promising therapeutic approach for geriatric disorders [16]. The expression of miR-9/9* and miR-124 (miR-9/9*-124) in human fibroblasts induces their conversion into neurons, a process facilitated by NEUROD2,
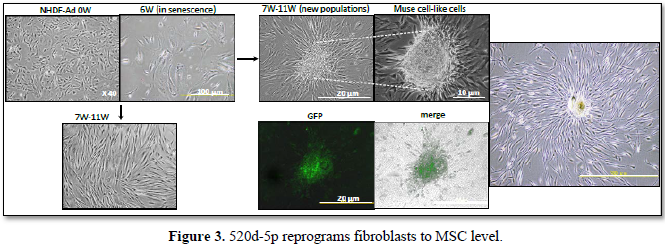
through compositional changes of SWI/SNF-like BAF chromatin-remodeling complexes [23]. Tissue repair and regeneration relies on the function of miRNAs, molecular silencers that enact post-transcriptional gene silencing of coding genes. Disruption of miRNA homeostasis is developmentally lethal, indicating that fetal tissue development is tightly controlled by miRNAs [24]. The human miRNA 520d-5p induced fibroblasts to Muse cell-like spheroid cells with CD105, Nanog, and P53 positive [2]. The authors presumed that it may be an adult type of human mesenchymal stem cell (hMSC), but it is the reason why the induced cells can easily differentiate and generate juvenile fibroblasts with CD105 positivity one after the other (Figure 3). Unlike dermal growth factor, this small ribonucleotide prevents hMSCs present in the skin being used up because it can mobilize them from differentiated to undifferentiated cell types.
In fibroblasts (NHDH-Ad: adult fibroblast cell line), 520d-5p induced the conversion to MSC and extension of the lifespan. After 520d-5p was transfected into cells of senescent status (6 weeks), the transfectants escaped apoptosis and survived as spheroid cells (Muse cell-like cells), resulting in the generation (7-11W & right) of juvenile fibroblasts from the spheroid cells until 24 weeks after the culture was started. Induction of the vector carrying 520d-5p was confirmed by the GFP expression. Safety was confirmed in vitro and we are planning applications for cosmetics.

CONVERSION FROM MALIGNANCY TO BENIGNANCY
Is it possible to convert a cancer cell to a healthy or original cell? Although at present it is difficult to revert a cancer cell to an original cell that lacks mutations in its genome, a benign cell with minimal mutations or methylation can be induced by miRNA [1]. Epigenetic alterations are involved in cancer initiation and progression but, unlike genetic mutations, the epigenetic state of cancer can be effectively reprogrammed via various approaches [25]. Interestingly, one miRNA has the potential to repair lethal DNA fragmentation, suggesting the ability to reset a complicated and convoluted genomic status [3]. We believe that we have a mission to search for miRNAs that enable the transformation into a close to healthy status, even if not the healthy status itself, with an eye to clinical applications, and 520d-5p is a prime candidate for this (Figure 4).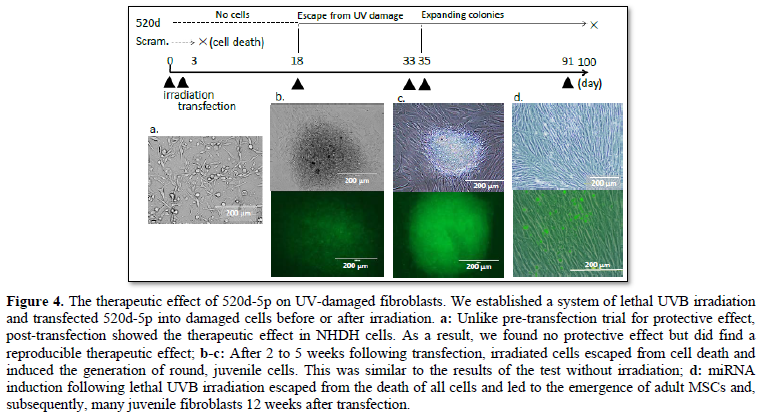
NGS ANALYSIS
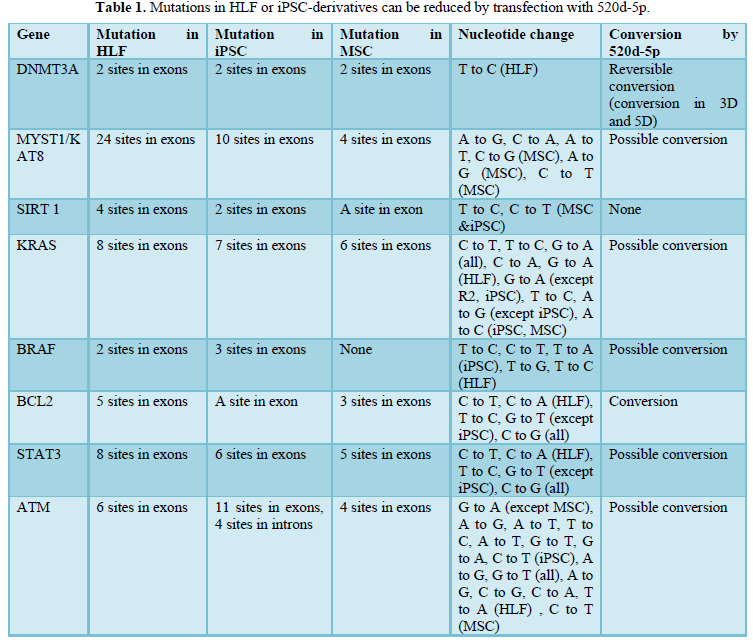
To estimate direct cellular reprogramming, and not just for miRNAs, NGS analysis is essential. Although we found a comprehensive analysis of miRNAs [26], no studies have used NGS analysis to examine the effects of miRNA, with the exception of our study. In our project using 520d-5p, we demonstrated a single nucleotide conversion to the wild type could be achieved using small RNA [4] (Figure 5) (Table 1). This technique can contribute to the qualitative improvement of induced pluripotent stem cells (iPSCs) with many genomic mutations or alterations. We expect the discovery of further miRNAs to make it possible to revert more than two nucleotides to the wild type resulting understanding its definite conversion rules.
APPLICATION
Small interfering RNA (siRNA) preparations are only a therapeutic modality using an adeno-associated viral vector as small RNA, and some preparations using adeno-associated virus (AAV) have already been used for clinical practice because of their very low pathogenicity, although the viral genome is incorporated into the host genome at a low frequency. miRNA with physiological functions in vivo, which makes it possible to replenish the deficiencies in cancer cells, has not yet been applied as a therapeutic strategy [27,28]. Applications for vaccine or siRNA vector targeting for virus (HPV, HBV, or HIV) have been designed and examined in vivo [29,3]. In terms of regeneration, miR-218-5p was notably upregulated in dermal papilla (DP) spheroid-derived exosomes. DP spheroid-derived exosomes upregulated b-catenin, promoting the development of hair follicles [30]. The manipulation of miRNA signalling holds great promise for regenerative medicine and aims to harness either endogenous or implanted cells to promote tissue repair [31]. The ability of miRNAs to regulate multiple targets might increase the efficacy of miRNA-based drugs, because the induction of miRNA is a replacement therapy that has an on-target effect, unlike siRNA which has an off-target effect. The total amount of miRNAs in humans at age 50 years is reduced to approximately half that at age 20. The proper supplementation of miRNAs is likely to have a positive effect on every part of the human body.
CONCLUSION
In the future, reports of useful miRNAs are likely to rapidly increase and will be examined in relation to clinical applications. Understanding the molecular and cellular pathways that are controlled by miRNAs, as illustrated for the main pathways of cancer development, may facilitate the development of miRNA-therapeutics. The clinical impact of miRNAs, including as biomarkers, identified in proof-of-concept studies in cell lines, animal models, and small patient-cohorts must be confirmed in carefully designed clinical studies. miRNAs themselves as preparations are unstable, and the challenge of stabilizing them remains unresolved, with another of the major hurdles in the way of making this application possible being the high cost of their synthesis. Also, innovative technical improvements in RNA synthesis are needed to realize the feasibility of miRNA-based medicine. We hope that this challenge will be overcome to translate this technology into future therapeutics.
- Tsuno S, Wang X, Shomori K, Miura K, Miura Y, et al. (2020) Conversion of human hepatoma cells by 520d-5p to benign or normal liver tissues via a stemness-mediated process. Oncogen 3: 22.
- Ishihara Y, Tsuno S, Ping B, Ashizaki T, Nakashima M, et al. (2016) Hsa-miR-520d-5p promotes survival in human dermal fibroblasts exposed to a lethal dose of UV irradiation. NPJ Aging Mech Dis 2: 16029.
- Ishihara Y, Tsuno S, Kuwamoto S, Yamashita T, Endo Y, et al. (2014) Hsa-miR-520d converts fibroblasts into CD105+ populations. Drugs R & D 14: 253-264.
- Miura N, Ishihara Y, Miura Y, Kimoto M, Miura K (2019) miR-520d-5p can reduce the mutations in hepatoma cancer cells and iPSCs-derivatives. BMC Cancer 19: 587.
- Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, et al. (2012) MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer. Cancer Discov 2: 1100-1108.
- Takayama KI, Misawa A, Inoue S (2017) Significance of microRNAs in androgen signalling and prostate cancer progression. Cancers 9: 102.
- Zedan AH, Blavnsfeldt SG, Hansen TF, Nielsen BS, Marcussen N, et al. (2017) Heterogeneity of miRNA expression in localized prostate cancer with clinicopathological correlations. PLoS One 12: e0179113.
- Cekaite L, Eide PW, Lind GE, Skotheim RI, Lothe RA (2016) MicroRNAs as growth regulators, their function and biomarker status in colorectal cancer. Oncotarget 7: 6476-6505.
- Chauhan R, Datzkiw D, Shrivastav SV, Shrivastav A (2018) Insilico identification of microRNAs predicted to regulate N-myristoyltransferase and Methionine Aminopeptidase 2 functions in cancer and infectious diseases. PLoS One 13: e0194612.
- Galoczova M, Coates P, Vojtesek B (2018) STAT3, stem cells, cancer stem cells and p63. Cell Mol Biol Lett 23: 12.
- Lin CP, Choi YJ, Hicks GG, He L (2012) The emerging functions of the p53-miRNA network in stem cell biology. J Cell Cycle 11: 2063-2072.
- Xing F, Liu Y, Wu SY, Wu K, Sharma S, et al. (2018) Loss of XIST in breast cancer activates MSN-c-Met and reprograms microglia via exosomal miRNA to promote brain metastasis. Cancer Res 78: 4316-4330.
- Ishihara Y, Tsuno S, Kuwamoto S, Yamashita T, Endo Y, et al. (2016) Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model. BMC Cancer 16: 415.
- Ishihara Y, Tsuno S, Kuwamoto S, Yamashita T, Endo Y, et al. (2017) Correction to: Tumor-suppressive effects of atelocollagen-conjugated hsa-miR-520d-5p on un-differentiated cancer cells in a mouse xenograft model. BMC Cancer 17: 666.
- Ishihara Y, Miura Y, Miura N, Miura K (2019) A novel anti-cancer effect of atelocollagen-conjugated miR-520d-5p on pancreatic cancer cells in vitro and in a mouse xenograft model. Integr Mol Med 6: 1-9.
- Watanabe K, Ikuno Y, Kakeya Y, Kito H, Matsubara A, et al. (2018) Functional similarities of microRNAs across different types of tissue stem cells in aging. Inflamm Regen 38: 9.
- McCubrey JA, Lertpiriyapong K, Steelman LS, Abrams SL, Yang LV, et al. (2017) Effects of resveratrol, curcumin, berberine and other nutraceuticals on aging, cancer development, cancer stem cells and microRNAs. Aging 9: 1477-1536.
- Kiss T, Giles CB, Tarantini S, Yabluchanskiy A, Balasubramanian P, et al. (2019) Nicotinamide mononucleotide (NMN) supplementation promotes anti-aging miRNA expression profile in the aorta of aged mice, predicting epigenetic rejuvenation and anti-atherogenic effects. Gero Science 41: 419-439.
- Balzano F, Cruciani S, Basoli V, Santaniello S, Facchin F, et al. (2018) MiR200 and miR302: Two big families influencing stem cell behavior. Molecules 23: 282.
- Zanotti S, Gibertini S, Blasevich F, Bragato C, Ruggieri A, et al. (2018) Exosomes and exosomal miRNAs from muscle-derived fibroblasts promote skeletal muscle fibrosis. Matrix Biol 74:77-100.
- Sinha M, Sen CK, Singh K, Das A, Ghatak S, et al. (2018) Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun 9: 936.
- Jayawardena TM, Egemnazarov B, Finch EA, Zhang L, Payne JA, et al. (2012) MicroRNA-mediated in vitro and in vivo direct reprogramming of cardiac fibroblasts to cardiomyocytes. Circ Res (2012) 110: 1465-1473.
- Yoo AS, Sun AX, Li L, Shcheglovitov A, Portmann T, et al. (2011) MicroRNA-mediated conversion of human fibroblasts to neurons. Nature 476: 228-231.
- Sen CK, Ghatak S (2015) miRNA control of tissue repair and regeneration. Am J Pathol 185(10): 2629-2640.
- Gong L, Yan Q, Zhang Y, Fang X, Liu B, et al. (2019) Cancer cell reprogramming: A promising therapy converting malignancy to benignity. Cancer Commun 39: 48.
- Srivastava D, DeWitt N (2016) In Vivo Cellular Reprogramming: The Next Generation. Cell 166: 1386-1396.
- Hardcastle N, Boulis NM, Federici T (2017) AAV gene delivery to the spinal cord: Serotypes, methods, candidate diseases, and clinical trials. Expert Opin Biol Ther 18: 293-307.
- Moore NA, Bracha P, Hussain RM, Morral N, Ciulla TA (2017) Gene therapy for age-related macular degeneration. Expert Opin Biol Ther 17: 1235-1244.
- Sato N, Saga Y, Uchibori R, Tsukahara T, Urabe M, et al. (2018) Eradication of cervical cancer in vivo by an AAV vector that encodes shRNA targeting human papillomavirus type 16 E6/E7. Int J Oncol 52: 687-696.
- Hu S, Li Z, Halle Lutz, Huang K, Su T, et al. (2020) Dermal exosomes containing miR-218-5p promote hair regeneration by regulating β-catenin signalling. Sci Adv 6: 30.
- Frith JE, Porrello ER, White J (2014) Concise review: New frontiers in MicroRNA-based tissue regeneration. Stem Cells Transl Med 3(8): 969-977.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cell Signaling & Damage-Associated Molecular Patterns
- Journal of Cardiology and Diagnostics Research (ISSN:2639-4634)
- Stem Cell Research and Therapeutics (ISSN:2474-4646)
- Journal of Spine Diseases
- Dermatology Clinics and Research (ISSN:2380-5609)
- International Journal of Anaesthesia and Research (ISSN:2641-399X)
- Journal of Renal Transplantation Science (ISSN:2640-0847)








