2725
Views & Citations1725
Likes & Shares
Rhabdomyolysis in pediatric patients presents unique challenges due to its varied etiology, clinical presentation, and management strategies. This paper discusses a case of an 8-year-old male with viral-induced rhabdomyolysis, characterized by severe myalgia, weakness, and dark urine. Laboratory investigations revealed markedly elevated creatine kinase levels and myoglobinuria, confirming the diagnosis. The patient was managed with aggressive fluid resuscitation, electrolyte monitoring, and supportive care, leading to a gradual resolution of symptoms and normalization of laboratory parameters.
Additionally, a literature review delves into the pathophysiology, clinical manifestations, and management strategies for rhabdomyolysis, particularly in pediatric patients. The etiology is multifaceted, including trauma, infections, drugs, toxins, and metabolic disorders, with approximately 25,000 cases reported annually in the United States. The pathophysiological mechanisms involve direct muscle injury or metabolic disturbances leading to intracellular calcium overload and subsequent muscle cell death.
Diagnosis relies on clinical examination and laboratory tests, notably elevated creatinine kinase levels. Although the management algorithm is not officially validated, it emphasizes fluid resuscitation, monitoring for complications, and establishing discharge criteria. ICU admission is crucial for severe cases with complications such as electrolyte imbalances or acute kidney injury.
Our discussion includes an algorithm for the management of rhabdomyolysis, highlighting the need for early detection, prompt intervention, and comprehensive follow-up to mitigate the risk of recurrent rhabdomyolysis and long-term kidney damage. Future research should focus on comparative studies to delineate optimal fluid maintenance strategies in pediatric rhabdomyolysis and enhance evidence-based management protocols.
Keywords: Rhabdomyolysis, Myoglobinuria, Creatinine Kinase, Acute kidney injury, Management strategies.
INTRODUCTION
Rhabdomyolysis is releasing skeletal muscle contents into the circulation when there is insult to it. These contents are responsible for the clinical presentation that the patient presents with. References to rhabdomyolysis can be traced back to biblical times. However, it wasn’t until the London bombings in World War II that the connection between rhabdomyolysis and acute kidney injury (AKI) was identified in victims of crush injuries [1]. This was followed by lot of other reports from other wars, one of them published in 1955, reported a mortality rate of approximately 80% to 90% was found in casualties with post-traumatic renal insufficiency in World War II and in the Korean war [2]. Until that time, only crush injuries was the reported as a cause of rhabdomyolysis, later it was found that nonphysical injuries can also result in rhabdomyolysis.
Despite the significant increase in incidence and the high mortality associated with rhabdomyolysis, there remains a lack of established guidelines for its management in both adult and pediatric populations. In this article, we present a pediatric case of viral-induced rhabdomyolysis and outline the algorithm we employed for its management. Our findings highlight the need for a comprehensive systemic approach to diagnosing rhabdomyolysis and determining the optimal timing for diagnostic tests. Additionally, in this review, we will explore the pathophysiology of rhabdomyolysis in depth, discuss its clinical manifestations, and outline management strategies for the syndrome.
CASE PRESENTATION SECTION
Patient Presentation
The patient presented with tactile fever, dry cough, vomiting (including one episode of blood-tinged vomit), and non-bloody, non-bilious loose stools with poor feeding.
Symptoms progressed to bilateral knee pain, difficulty walking, and proximal upper limb weakness. Initially treated with antibiotics and antihistamine at a private clinic, with no improvement. Tested positive for influenza A upon arrival at our hospital's emergency department.
Physical Examination
The patient appears lethargic but alert, with normal growth parameters (Table 1). Capillary refill time is less than 2 seconds, with sunken eyes and normal conjunctiva. Warm, dry, pink skin without rashes. Normocephalic with mildly erythematous tympanic membranes and moist oral mucosa. No scleral icterus or lymphadenopathy. Lung and cardiac examinations are normal. Abdomen is soft, mildly tender, without distention or organomegaly. Severe pain on limb movement with 2/5 power in lower limbs and 3/5 in upper limbs. Brisk deep tendon reflexes and impaired gait due to pain.

Investigation
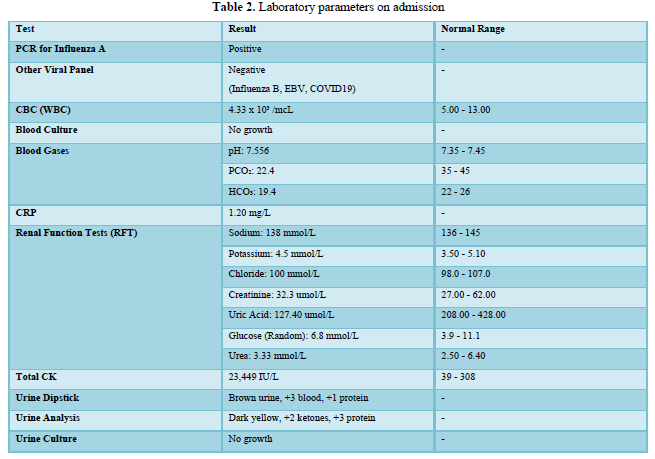
Upon admission, the patient tested positive for influenza A through PCR, while other viral panels returned negative. The complete blood count indicated a low white blood cell count, and blood cultures showed no growth, reinforcing the viral etiology.
Blood gas analysis revealed respiratory alkalosis, and C-reactive protein levels suggested mild inflammation. Renal function tests confirmed normal kidney function, but total serum creatine kinase levels were significantly elevated, indicating muscle breakdown.
Urine analysis showed dark urine with blood and protein but ruled out urinary tract infection through negative cultures. These findings culminated in a provisional diagnosis of viral-induced rhabdomyolysis, leading to the patient's admission for further management. Table 2 shows the laboratory parameters on admission.
Progress Through Admission
Creatine kinase (CK) levels were closely monitored, demonstrating a clear improvement starting on the second day of hospitalization. This positive trend coincided with a change in fluid management, where the maintenance rate was increased from 1.5 times to double the maintenance. The early adjustments in fluid therapy contributed significantly to the gradual reduction of CK levels, indicating effective management of the patient's condition.
Throughout the admission, the medical team focused on supportive care, including electrolyte monitoring and fluid resuscitation, which played a crucial role in the patient’s recovery. The management strategies implemented during the hospitalization effectively addressed the complications associated with viral-induced rhabdomyolysis, leading to a favorable clinical outcome. Figure 1 traces the level of creatinine kinase throughout admission.

DISCUSSION
Epodemology
Every year in the United States, there are around 25,000 reported instances of rhabdomyolysis. Over a span of three years at the University of California, the occurrence rate of rhabdomyolysis in children was found to be 0.26%, which equates to 4 out of every 1,500 inpatient consultations. The occurrence of acute kidney injury, a possible complication of rhabdomyolysis, ranges from 5% to 30%. However, the reported rates of acute kidney injury (AKI) in the context of rhabdomyolysis can vary widely. This is due to the different ways AKI is defined and the varying degrees of rhabdomyolysis severity [3,4].
Etiology
Infections are often identified as the primary cause of rhabdomyolysis in children. Specifically, over a third of pediatric rhabdomyolysis cases are attributed to viral infections. Viruses such as Influenza, Epstein-Barr, and Cytomegalovirus have been frequently identified as culprits. Bacterial infections, including Group A streptococci and Salmonella, are also documented causes in children, according to pediatric literature. Furthermore, protozoal infections like Malaria can also be responsible. While trauma is the predominant cause of rhabdomyolysis in adults, it’s less prevalent in children. However, there’s been a noted increase in the incidence of exertional rhabdomyolysis among teenagers. Medications such as colchicine, statins, daptomycin, corticosteroids, amphetamines, zidovudine, fibrates, diuretics, antimalarials, anticholinergics, intravenous and intramuscular illicit drug use has been reported as a possible trigger to rhabdomyolysis. Inherited disorders and other myopathies also can result in rhabdomyolysis and is usually a recurrent history of rhabdomyolysis rather than a single attack. G.D. Giannoglou listed the causes of rhabdomyolysis in Table 3 [5,6,7].

Pathophysiology
Although it’s not fully understood until now but getting to know the pathophysiology behind rhabdomyolysis helped in forming a rough management plan for the affected patients. Figure 2 shows the pathophysiology behind rhabdomyolysis. It starts with one of two ways; the first one is by a direct insult to the skeletal muscle membrane. This leads to entry of calcium (Ca+) to the cell which increases its concentration intracellularly. The increase of calcium activates two enzymes, Protease and Phospholipase, these two causes destruction to the cell membrane and to the mitochondria. Further destruction via the enzymes causes more entry of calcium to the cell [5,8].
Sometimes a depletion of the energy can be the cause of rhabdomyolysis. It can happen either because of an increase in consumption or decrease in production. With no energy available there will be inhibition of the action of the Sodium Potassium (3Na/2K) ATPase Pump due to deficiency in the ATP. That lead to Increase in the intracellular sodium level (Na) which in turn activate the Sodium Calcium (Na/Ca) Pump that push the sodium outside the cell under its concentration gradient and pushes the calcium to the cell. That leads to an increase in the intracellular calcium level and the cascade mentioned in the previous paragraph continues to happen. Protease and Phospholipase, the two enzymes mentioned previously, are also responsible for the energy depletion via destruction of the mitochondria in the skeletal muscle [5,8].
An inhibitory pathway usually happens to counteract the rise in the calcium level. This pathway is carried out by Calcium ATPase Pump, this pump senses the level of the calcium in the cell and acts to decrease it by pushing it outside the cell. However, due to the energy depletion in rhabdomyolysis patients this process can’t be carried out as it needs ATP to function. As a result, calcium level will keep increasing which then leads to skeletal muscle repeated contractions until it dies. The death of the skeletal muscle leads to release of its content to the circulation. These contents cause the clinical presentation of the patients with rhabdomyolysis [5].

After muscle cell death occurs, the intracellular contents are released into the extracellular space and circulation. These released contents contribute to the clinical manifestations observed in rhabdomyolysis, distinguishing it from myositis. The impact extends to surrounding tissues, leading to local vasculature damage, edema, elevated compartmental pressure, and ischemia. A detailed overview of specific contents, their systemic effects, and their correlation with clinical findings is presented in Table 4 [5].

Clinical Presentation
The clinical triad of rhabdomyolysis comprises myalgia, muscle weakness, and tea-colored urine. However, numerous pediatric cases have reported myalgia as the sole symptom of rhabdomyolysis. Additional symptoms related to the underlying etiology can also be present, providing clues to the trigger of rhabdomyolysis. Specifically, for inborn errors of metabolism, the onset of muscle pain is very important to know the specific disease. Muscle pain that occurs minutes after moderate to high-intensity exercise is a typical presentation of Glycogen Storage Disease. If the pain subsides after a rest period of 6 to 10 minutes, this scenario is indicative of McArdle Disease. Weakness and stiffness following low intensity, but prolonged exercise is usually due to Fatty Acid Oxidation Defect. Lastly, Mitochondrial Diseases typically present with myalgia following a period of fasting. It is crucial to inquire about the symptoms of acute kidney injury to rule out potential complications [5,9].
Diagnosis
A management algorithm for rhabdomyolysis patients can be formulated based on a comprehensive review of rhabdomyolysis cases documented over the previous decade. The initial step involves a thorough physical examination of the patients, with a critical emphasis on vital signs to ascertain the immediate need for emergency intervention. It is paramount to perform an examination to exclude the possibility of compartment syndrome, which could either be a precipitating factor or a subsequent complication of rhabdomyolysis. In instances where the limb exhibits signs of compartment syndrome, immediate surgical consultation becomes a necessity. A comprehensive neurological examination is instrumental in identifying the muscles impacted by rhabdomyolysis. Essential aspects of the examination include gait observation and assessment of muscle tone and strength. Additional specific examinations may be warranted, contingent on the suspected etiology or trigger of the rhabdomyolysis. For instance, in the event of a suspected viral upper respiratory tract infection (as in our case), a throat examination would be advised. Similarly, spleen examination would be necessary if Epstein-Barr Virus (EBV) infection is suspected, and a mental status examination would be required in the event of suspected toxin injection [7].
Moving to the diagnosis, the patient should be sent to the emergency department to carry emergency lab tests to confirm the diagnosis of rhabdomyolysis when it’s suspected from the history and physical examination. The collection of blood samples should not be postponed under any circumstances to avoid any delay in the management of the condition. Complete blood count (CBC) is important to check the leukocyte level if it’s high, that raises the suspicion of infection trigger. The thrombocyte count can also indicate if disseminated intravascular coagulation (DIC) has occurred as a complication of rhabdomyolysis. Serum creatinine kinase (CK) is the most sensitive lab test to diagnose a patient with rhabdomyolysis. There is no consensus on the cutoff value in pediatric or adult patients, but most consider more than 5 times the upper limit of normal (or 1,000 U/) diagnostic. It’s very important to know the half-life of creatinine kinase because it may help in predicting when will the value start declining. Figure 3 by G.D. Giannoglou compares the half-life of creatinine kinase to myoglobin, explaining why myoglobin (which can be measured from the blood or urine) is not widely used to diagnose rhabdomyolysis due to its short half-life leading to high false-negative results [7].

Conducting a renal function test remains of paramount importance to establish a baseline for electrolytes and creatinine levels. This serves to monitor kidney function, given that acute kidney injury is the most consequential and severe complication that can arise from rhabdomyolysis. As previously noted, potassium levels in these patients can be life-threatening, as they can precipitate fatal arrhythmias. Electrocardiogram (ECG) showed be done in any case where there are electrolytes disturbances [5].
Dipstick analysis of urine samples typically yields a positive result for blood. However, upon microanalysis, it is revealed that this ‘blood’ is in fact myoglobin, not red blood cells (RBC). Evaluating nitrate and leukocyte esterase levels can be beneficial in excluding urinary tract infections. Occasionally, a urine culture may be warranted to definitively diagnose and subsequently treat urinary tract infections [10].
Additional diagnostic testing for the underlying trigger should be considered, particularly if there is a suspicion of ongoing rhabdomyolysis. Polymerase Chain Reaction (PCR) for viral causes, urine toxicology for drugs and toxins, and medication serum level checks can be recommended to ascertain the cause of the rhabdomyolysis. Neurological tests such as electromyography and muscle MRI typically yield nonspecific findings in terms of myopathy and inflammation. These tests are only beneficial if conducted when the patient is asymptomatic. Muscle biopsy, on the other hand, is not a useful diagnostic test in an acute setting; it should be performed six weeks post-insult. Genetic testing is only required when the patient has recurrent rhabdomyolysis or a positive family history of similar episodes and should only be conducted once the patient becomes asymptomatic. In patients with recurrent exercise-induced rhabdomyolysis, a forearm exercise test can be performed to screen for metabolic myopathy by examining lactic acid and ammonia levels before and after the placement of a sphygmomanometer cuff. [10].
Treatment & Prognosis
A treatment algorithm for pediatric patients with rhabdomyolysis has been developed based on reported cases and supporting evidence for the management of this condition. While it is not an officially verified algorithm, it is a proposal that we have utilized in our facility to manage this complex condition. The algorithm is depicted in Figure 4.

Prior to initiating management, it is crucial to identify emergent situations that necessitate admission to the Intensive Care Unit (ICU). These include severe electrolyte disturbances, the presence of arrhythmias, or acute kidney injury requiring dialysis. In the absence of these red flags, the patient should be admitted to the ward for fluid management and monitoring [3,4].
Beginning with the choice of fluid, there is no empirical evidence favoring the use of Ringer’s Lactate over Normal Saline (NS). Based on case reports, the majority of patients were treated with Normal Saline. The addition of Sodium Bicarbonate is only indicated in the presence of acidosis. There is no comprehensive literature review that either supports or contradicts the use of mannitol in the treatment of pediatric rhabdomyolysis [3,4].
In pediatric patients, fluid administration commences with a bolus of 20ml/kg, followed by a transition to double maintenance. Additional boluses can be administered as required. In the presence of cardiac complications or acute kidney injury, consultation with a pediatric cardiologist and/or nephrologist is advised for potential adjustment of the fluid regimen to 1.5x maintenance. The efficacy of the treatment should be gauged by monitoring clinical improvement and the patient’s urine output. The target urine output is set at 3 to 4 times the normal urine output, equating to 3-4ml/kg/hr. If the patient fails to achieve this output or exhibits signs of acute kidney injury, consultation with a pediatric nephrologist is recommended [4,5].
Evaluation for potential complications of rhabdomyolysis is crucial. In the event of Compartment Syndrome, consultation with a pediatric surgeon is advised. In cases of Disseminated Intravascular Coagulopathy (DIC), a pediatric hematologist should be consulted for the administration of fresh frozen plasma [8].
Monitoring of creatinine kinase and renal function tests should be conducted at least every two days to track the patient’s progress. Discharge should only be considered when the following criteria are met: the patient is asymptomatic, with normal electrolyte levels, normal creatinine and renal function tests, and demonstrates a good urine output indicative of a decreasing trend in creatinine kinase levels. No patient should be discharged if the creatinine kinase level is equal to or exceeds 5,000 U/L, as this is the minimum level of CK associated with Acute Kidney Injury (AKI) [4].
A follow up appointment should be scheduled three days post-discharge in the outpatient department (OPD) if the creatinine kinase levels were not normal at the time of discharge. In cases where there is a positive family history of rhabdomyolysis or recurrent episodes of rhabdomyolysis (more than one episode), the patient should be referred to the Neurology Clinic & Genetics Clinic for further evaluation of potential underlying conditions [5].
CONCLUSION
In conclusion, this paper emphasizes the complexities of diagnosing and managing rhabdomyolysis in pediatric patients, as demonstrated by the case of an 8-year-old boy with viral-induced rhabdomyolysis. The unique clinical presentation and multifactorial etiology necessitate a high index of suspicion and a systematic approach to management. Our findings underscore the importance of early diagnosis, aggressive fluid resuscitation, and careful monitoring of laboratory parameters to ensure favorable outcomes and prevent complications such as acute kidney injury.
The literature review highlights that rhabdomyolysis can arise from a variety of causes, particularly viral infections in children. Pathophysiological mechanisms involve intricate cellular processes that lead to muscle injury and the subsequent release of myoglobin and other muscle contents into circulation. Establishing a clear management algorithm, as outlined in this study, is crucial for guiding healthcare providers in treating this condition effectively.
Future research should aim to explore optimal fluid management strategies and establish evidence-based protocols to enhance the understanding and treatment of pediatric rhabdomyolysis. This approach will ultimately contribute to improving patient care and outcomes in this vulnerable population.
-
- Bywaters, E. G., & Beall, D. (1998). Crush injuries with impairment of renal function. Journal of the American Society of Nephrology, 9, 322–332.
- Smith, L. H. Jr., Post, R. S., Teschan, P. E., Abernathy, R. S., Davis, J. H., Gray, D. M., Howard, J. M., Johnson, K. S., Klopp, E., Mundy, R. L., O’Meara, M. P., & Rush, B. F. Jr. (1955). Post-traumatic renal insufficiency in military casualties. II. Management, use of an artificial kidney, prognosis. American Journal of Medicine, 18, 187–198.
- Melli, G., Chaudhry, V., & Cornblath, D. R. (2005). Rhabdomyolysis: an evaluation of 475 hospitalized patients. Medicine (Baltimore), 84(6), 377-385.
- Chamberlain, M. C. (1991). Rhabdomyolysis in children: a 3-year retrospective study. Pediatric Neurology, 7, 226–228.
- Szugye, H. S. (2020). Pediatric Rhabdomyolysis. Pediatrics in Review, 41(6), 265–275. https://doi.org/10.1542/pir.2018-0300
- Luetmer, M. T., Boettcher, B. J., Franco, J. M., Reisner, J. H., Cheville, A. L., & Finnoff, J. T. (2019). Exertional Rhabdomyolysis. Medicine & Science in Sports & Exercise, 1. https://doi.org/10.1249/mss.0000000000002178
- Giannoglou, G. D., Chatzizisis, Y. S., & Misirli, G. (2007). The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. European Journal of Internal Medicine, 18(2), 90–100. https://doi.org/10.1016/j.ejim.2006.09.020
- Mannix, R., Tan, M. L., Wright, R., & Baskin, M. (2006). Acute pediatric rhabdomyolysis: causes and rates of renal failure. Pediatrics, 118, 2119–2125.
- Poels, P. J. E., & Gabreels, F. J. M. (1993). Rhabdomyolysis: a review of the literature. Clinical Neurology and Neurosurgery, 95, 175–92.
- Beetham, R. (2000). Biochemical investigation of suspected rhabdomyolysis. Annals of Clinical Biochemistry, 37, 581–7.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)






