8917
Views & Citations7917
Likes & Shares
Systemic inflammatory response syndrome (SIRS) is a multiplex pathophysiological defense response against noxious stressor such as infection, trauma, burns, or any others injuries. Study objective was to evaluate the antioxidants and anti-inflammatory potential of Biofield Energy Treated (Blessed) Proprietary Test Formulation and Biofield Energy Healing Treatment (Blessing) per se to the animals on Cecal Slurry, LPS, and E. coli-induced SIRS model in Sprague Dawley rats. Each component of the test formulation was divided into two parts; one part was denoted as untreated test formulation, while other part of the test formulation and three group of animals received Biofield Energy Healing Treatment remotely for about 3 minutes by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The level of MPO was significantly (p≤0.001) reduced by 51.44%, 71.69%, 55.79%, 55.16%, and 58.12% in G5 (Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15); G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated/Blessed test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation) groups, respectively with reference to disease control (G2) group. The level of LPO end product in terms of malondialdehyde (MDA) was significantly (p≤0.001) reduced by 52.71%, 56.54%, 67.35%, and 75.28% in G6, G7, G8, and G9 as compared to the G4 group. The level of MMP-9 was significantly (p≤0.001) decreased by 34.79%, 48.57%, 39.29%, and 41.25% in G6, G7, G8, and G9, respectively with reference to G4 group. Moreover, the level of FDP was significantly (p≤0.001) decreased by 39.87%, 44.91%, 39.76%, 43.09%, and 46.47% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the G2 group. The level of substance P was significantly (p≤0.001) decreased by 19.93%, 25.51%, and 27.92% in the G7, G8, and G9 groups, respectively as compared to the G4 group. The level of iNOS was significantly (p≤0.001) decreased by 39.26% (p≤0.001), 38.95% (p≤0.001), 47.63% (p≤0.001), and 59.78% (p≤0.001) in the G6, G7, G8, and G9 groups, respectively as compared to the G2 group. Overall, the data suggested the antioxidant and anti-inflammatory potentials of the Biofield Energy Treated test formulation and Biofield Energy Treatment per se with respect to various inflammatory conditions that might be beneficial various types of systemic inflammatory disorders specially sepsis, trauma, septic shock or any types of injuries. Consequently, the results significantly slowdown the inflammation-related symptoms in preventive treatment groups like G6, G7, G8, and G9.
Keywords: SIRS, Biofield treatment, Antioxidant, Inflammatory biomarkers, The Trivedi Effect®, ELISA
INTRODUCTION
Systemic inflammatory response syndrome (SIRS) is a multiplex pathophysiologic defense response of the body to a noxious stressor such as infection, trauma, burns, pancreatitis, surgery, acute inflammation, ischemia or reperfusion, or malignancy or any others injuries [1,2]. Sepsis is an infection which can considered a systemic inflammatory response. Clinically, the SIRS is identified by two or more symptoms including fever or hypothermia, tachycardia, tachypnoea and change in blood leucocyte count [3]. The progression from sepsis to “septic shock” causes high rate of mortality. Research in the last two decades explored that the inflammatory process is play a major role in the mechanism of different vital systems pathologies [4]. Matrix metalloproteinases (MMPs) are are zinc-dependent endopeptidase enzymes, responsible for tissue remodeling in both physiological and pathophysiological conditions [5]. Fibrin degradation products (FDP) are the components of blood produced by clot degeneration. In normal subjects, the plasma FDP levels are not detectable. When the levels are raised above 200 ng/mL, it can be detectable in the plasma. Besides, in response to inflammation, the body produces more fibrinogen and its degradation products [6]. Superoxide dismutase’s (SODs) is an important antioxidant enzyme acts against reactive oxygen species-mediated diseases [7]. The neuropeptide substance P (SP) is an 11 amino acid peptide distributed throughout the nervous system of human and animal species. SP has a potent neuroimmunomodulator actions through mediation of neurokinin-1 receptor and proinflammatory effects in vitro and in vivo, and also influence many immune and inflammatory disorders [8,9]. There is increasing evidence that nitric oxide (NO) is an important factor in the pathogenesis of septic shock. According to Tsukahara et al. reported that the mRNA expression of inducible NO synthase (iNOS) has increased in both sepsis and SIRS cases, which measured in terms of polymorphonuclear neutrophils (PMNs) by reverse transcriptase polymerase chain reaction (RT-PCR) method [10]. Therefore, to study the alteration of antioxidants and inflammatory biomarkers in lungs and liver tissues in presence of Cecal Slurry, LPS and E. coli-induced SIRS model in Sprague Dawley (SD) rats. In this circumstance, a novel test formulation was designed with the combination of vital minerals (selenium, zinc, iron, calcium, and magnesium), essential vitamins (cyanocobalamin, ascorbic acid, pyridoxine HCl, vitamin E, and D3), and nutraceuticals (Ginseng, cannabidiol-CBD isolate, and β-carotene). All the vitamins and minerals used in the test formulation have significant functional role to provide vital physiological responses [11,12]. Besides, CBD itself shows wide pharmacological activities and reported in different types of disorders [13,14]. Ginseng extract is one of the excellent immune boosters to maintain overall immune response [15]. The current study was aimed to investigate the antioxidant and anti-inflammatory potential of Biofield Energy Treated/Blessed Proprietary Test Formulation and Biofield Energy Healing Treatment/Blessing per se to the animals on Cecal Slurry, LPS and E. coli-induced SIRS model in SD rats.
Biofield Energy Healing/Blessing Treatment or Biofield Therapy has been widely reported with significant impact against various diseases, and considered as one of the Complementary and Alternative Medicine (CAM) treatment approach [16-18]. National Center for Complementary and Alternative Medicine (NCCAM) recommended CAM with several clinical benefits with reference to conventional therapy [19]. National Centre of Complementary and Integrative Health (NCCIH) accepted Biofield Therapy as a CAM health care approach in addition to other therapies such as Tai Chi, deep breathing, yoga, natural products, Johrei, therapeutic touch, pranic healing, Reiki, chiropractic/osteopathic manipulation, guided imagery, meditation, movement therapy, special diets, massage, hypnotherapy, homeopathy, relaxation techniques, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems [20, 21]. The Trivedi Effect® was scientifically reported on various disciplines viz. materials science [22, 23], agriculture science [24], microbiology [25, 26], biotechnology [27], and improved bioavailability of various compounds [28, 29], skin health [30, 31], nutraceuticals [32], cancer research [33], bone health [34, 35], overall human health and wellness. In this study, the authors want to evaluate the impact of Biofield Energy Healing/Blessing (prayer) Treatment (the Trivedi Effect®) on novel proprietary test formulation and to the animals per se by analyzing liver and lungs biomarkers in presence of Cecal Slurry, LPS and E. coli-induced SIRS model in Sprague Dawley rats using standard ELISA assay.
MATERIALS AND METHODS
Chemicals and Reagents
Pyridoxine hydrochloride, vitamin (vit.) B6, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, provit A) were purchased from TCI, Japan. Vit. B12, calcium chloride, vit. E, vit. D3, iron (II) sulphate, and carboxymethyl cellulose sodium (CMC-Na) were procured from Sigma-Aldrich, USA. Sodium selenate and vit. C were obtained from Alfa Aesar, India. Panax ginseng extract and cannabidiol (CBD) isolate were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Dexamethasone was obtained from Clear synth, India. For the estimation of antioxidant and inflammatory biomarker panel in the lungs (MMP-9, FDP, Substance P, iNOS), and in liver such as MPO, SOD, and LPO were procured from CUSABIO, USA using specific ELISA kits.
Maintenance of Animals for Experiment
The male Sprague Dawley (SD) rats with body weight (200 to 300 gm) were obtained from M/s. Vivo Bio Tech, Hyderabad, India. Animals were kept in sterilized cages made up with polypropylene and stainless-steel top grill having feature for pellet feed and drinking water bottle that are fitted with stainless steel sipper tube. As per standard protocol all the animals were maintained throughout the experimental period.
Biofield Energy Healing (Blessing) Strategies
Each ingredient of the novel proprietary test formulation was divided into two parts. One part of each ingredient did not receive any treatment/Blessing and defined as untreated. The other part of each ingredient was treated with the Trivedi Effect® - Energy of Consciousness Healing Treatment (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for about 3 minutes. Besides, three group of animals were also received Biofield Energy Healing Treatment by Mr. Mahendra Kumar Trivedi under same laboratory conditions for about 3 minutes. The Biofield Energy Healer was located in the USA; however, the test formulation was located in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission/Blessing (prayer) was done to the tested samples or animals remotely for about 3 minutes via online web-conferencing platform. Thenceforth, the Biofield Energy Treated/Blessed samples were kept in a sealed condition for experiment. Similarly, the control (untreated) test formulation was subjected to “sham” healer for about 3 minutes treatment, under the same laboratory conditions. The “sham” healer, a person who did not have any knowledge about the Biofield Energy Treatment/Blessing. The Biofield Energy Treated animals were also taken back to the experimental room.
Study Design
As per study plan the experiment were designed into nine groups based on their body weight consisted 10-12 animals in each group. Group (G1) defined as normal control (vehicle, 0.5% w/v CMC-Na), group (G2) denoted as disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na), group (G3) referred as reference item (Cecal Slurry, LPS and E. coli + Dexamethasone), group (G4) included Cecal Slurry, LPS and E. coli along with untreated test formulation, group (G5) as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation, group (G6) defined as Cecal Slurry, LPS and E. coli + Biofield Energy Healing Treatment/Blessing per se to animals from day -15, group (G7) as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15, group (G8) included Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se Biofield Energy Treated test formulation from day -15, and group (G9) denoted as Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation.
Experimental Procedure
The animals were randomized and assigned to different groups based on the body weight after acclimatization for seven days. Just before dosing the test formulation were prepared and administered to the animal’s dose volume @10 mL/kg both morning and evening with the help of oral intubation graduated disposable syringe. Groups assigned to G1 to G5, and G9 animals were dosed with respective test formulation from day 1 to the end of the experiment. Groups assigned to G7 and G8 were dosed from day -15 and continued till end of the experiment. However, Group G6 animals received Biofield Energy Healing Treatment/Blessing on day -15. At 8th week, the animals were sacrifice and lungs and liver were collected, homogenised, and the supernatant subjected for estimation of antioxidants in liver (MPO, SOD, and LPO) and others biomarkers in lungs (MMP-9, FDP, Substance P, iNOS).
Induction of Systemic Inflammatory Response Syndrome (SIRS) Model
A combination model of sepsis was developed in SD rats by administering Cecal slurry (from donor animals, intraperitoneally, at the dose of 400 mg/kg) in combination with LPS (at the dose of 100 µg/animal) and E. coli [Escherichia coli; 0.2 mL (2M CFU)/animal]). The animals were monitored for various parameters for up to 56 days after disease (SIRS) induction. Ten Donor (~20 weeks old) rats were anesthetized. A midline laparotomy was performed on them and the cecum was extruded. A 0.5 cm incision was made on the anti-mesenteric surface of the cecum, and the cecum was squeezed to expel the feces. The feces from different donor animals were collected and weighed. Immediately after collection, the feces were pooled, diluted 1:3 with 5% dextrose solution and filtered to get a homogeneous suspension. Bacterial viability in the cecal slurry was analyzed. Cecal slurry prepared from donor rats was injected intraperitoneally into experimental rats (G2 to G9) at the dose of 400 mg/kg within 2 hours of preparation. After 3 hours, lipopolysaccharide (LPS) at the dose of 100 µg/animal, and gram-negative viable bacteria such as E. coli [0.2 mL (2M CFU)/animal] were injected, intraperitoneally (G2 to G9).
Preparation of Sample for the Estimation of Antioxidant and Other Biomarkers
With the continued treatment to the respective groups of 8th week of the experimental period, all the animals were sacrificed, lungs and liver were collected and stored at -20°C, homogenized and subjected for the estimation of antioxidants.
Estimation of Antioxidants and Other Biomarkers Levels
The liver from all the groups was subjected for the estimation of level of antioxidants such as MPO (CSB-E08722r), SOD (706002), and LPO (700870) and other vital biomarkers in lungs such as MMP-9 (CSB-E08008r), FDP (CSB-E07942r), Substance P (CSB-E08358r), iNOS (CSB-E08325r). All the biomarkers were estimated using ELISA as per manufacturer’s instructions.
Statistical Assessment
The obtained study data were shown as mean ± standard error of mean (SEM). Sigma-Plot statistical software (Version 11.0) was utilized for the analysis of data. Between two groups comparison Student’s t-test was used; while One-way analysis of variance (ANOVA) for multiple analysis, followed by post-hoc analysis, Dunnett’s test was used. The p≤0.05 was considered as the cut-off value for statistically significant.
RESULTS AND DISCUSSION
Assessment of Antioxidants in Liver Homogenate
Estimation of Myeloperoxidase (MPO)
Myeloperoxidase (MPO), was estimated in the presence of the test formulation and the data are graphically shown in Figure 1. The data suggested that the disease control (Cecal Slurry + LPS + E. coli + 0.5% CMC-Na) group (G2) showed the value of MPO as 57.78 ± 5.87 ng/mL, which was increased by 180.92% as compared with the normal control (G1, 20.57 ± 1.12 ng/mL). However, positive control (Dexamethasone) treatment (G3) showed the level of MPO i.e., 26.27 ± 2.43 ng/mL, which was significantly (p≤0.001) decreased by 54.54% as compared to the G2 group. The level of MPO in liver was significantly (p≤0.001) decreased by 59.69%, 51.44%, 71.69%, 55.79%, 55.16%, and 58.12% in the G4 (Cecal Slurry, LPS and E. coli along with untreated test formulation); G5 (Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS + E. coli + Biofield Energy Treated test formulation from day -15); G8 (Cecal Slurry, LPS + E. coli + Biofield Energy Treatment per se + Biofield Energy Treated/Blessed test formulation from day -15), and G9 (Cecal Slurry + LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation) groups, respectively with reference to disease control (G2) group. On the other hand, the level of MPO was also reduced by 29.77% in the G6 as compared to the untreated test formulation (G4). Myeloperoxidase (MPO) is released by activated neutrophils, characterized by powerful pro-oxidative and proinflammatory properties [36]. Overall, Mr. Trivedi’s Blessing (the Trivedi Effect®) remarkably reduced the level of MPO which might be helpful for the management of various inflammatory diseases.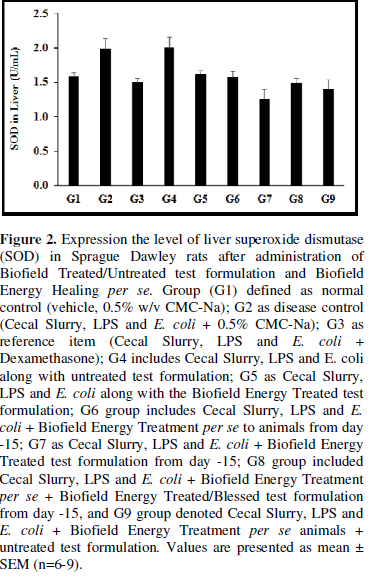
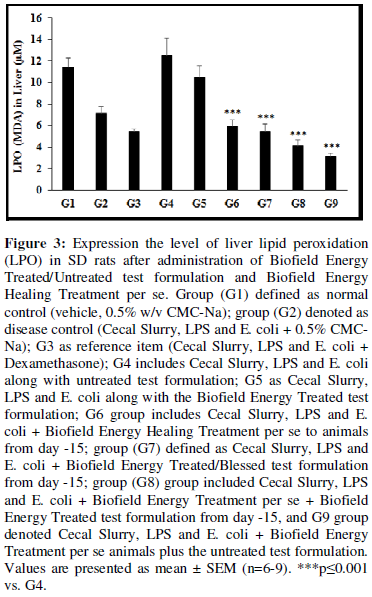
Estimation of lipid peroxidation (LPO)
The level of lipid peroxidation (LPO) end product in terms of malondialdehyde (MDA) was detected in all the experimental groups and the data are shown in Figure 3. The disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) group (G2) showed value of MDA as 7.17 ± 0.62 µM. While, the positive control (Dexamethasone) treatment (G3) decreased the level of MDA by 24.04% i.e. 5.45 ± 0.25 µM as compared to the G2 group. The level of MDA was significantly decreased by 17.36%, 24.05%, 42.93%, and 56.80% in the G6 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se to animals from day -15); G7 as Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15; G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated/Blessed test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation) groups, respectively with reference to G2 group. Moreover, the level of MDA was significantly reduced by 16.18%, 52.71% (p≤0.001), 56.54% (p≤0.001), 67.35% (p≤0.001), and 75.28% (p≤0.001) in G5, G6, G7, G8, and G9, correspondingly with reference to untreated test formulation (G4) group. Chronic inflammation can induce oxidative/nitrosative stress and lipid peroxidation (LPO), and its produce more reactive oxygen species (ROS), reactive nitrogen species (RNS), and DNA-reactive aldehydes and damaged the DNA in the cells [39]. DNA damage by lipid peroxidation products can leads to cancer [40]. Overall, Biofield Energy Treated test formulation and Biofield Energy Treatment per se reduced the level of lipid peroxidation (LPO) end product in terms of malondialdehyde (MDA), which could reduce the oxidative free radical and ultimately chances of less inflammation.
Assessment of Cytokines in Lungs Homogenate
Estimation of Matrix Metallopeptidase 9 (MMP-9)
Expression the level of lungs matrix metallopeptidase 9 (MMP-9) after administration of Biofield Treated test formulation and Biofield Energy Treatment to the Sprague Dawley rats, and the results are graphically presented in the Figure 4. The disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) group (G2) showed value of MMP-9 as 305.01 ± 28.92 pg/mL, which was increased by 64.94% as compared with the normal control (G1, 184.93 ± 6.29 pg/mL). Further, the positive control (Dexamethasone) treatment (G3) group decreased MMP-9 level by 35.31% i.e., 197.30 ± 20.08 pg/mL as compared to the G2 group. The level of MMP-9 was decreased by 17.82%, 33.27%, 47.36%, 37.87%, and 39.88% in the G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15); G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation) groups, respectively with reference to disease control group (G2). Besides, the level of MMP-9 was significantly reduced by 19.69%, 34.79% (p≤0.001), 48.57% (p≤0.001), 39.29% (p≤0.001), and 41.25% (p≤0.001) in G5, G6, G7, G8, and G9, correspondingly with reference to untreated test formulation (G4). MMP-9 plays a crucial role in immune cell function and acts as modulators of inflammation. The expression of MMP-9 is upregulated during inflammatory conditions like arthritis, diabetes, and cancer [41, 42]. Here, Mr. Trivedi’s Blessing (the Trivedi Effect®) has significantly reduced the level of MMP-9, which could be beneficial to combat inflammatory disease conditions.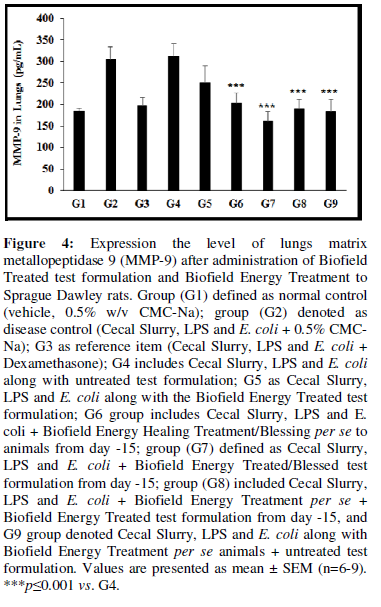
Estimation of Fibrin Degradation Products (FDP)
Expression the level of lungs fibrin degradation products (FDP) after administration of Biofield Treated test formulation and Biofield Blessing to Sprague Dawley rats, and the results are graphically presented in Figure 5. The disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) group (G2) showed value of FDP as 733.80 ± 26.28 ng/mL, which was increased by 418.59% as compared with the normal control (G1, 141.5 ± 2.66 ng/mL). Further, the positive control (Dexamethasone) treatment (G3) showed a significant (p≤0.001) decrease the level of FDP by 30.83% i.e., 507.57 ± 28.12 ng/mL as compared to the G2 group. The level of FDP was significantly (p≤0.001) decreased by 39.60%, 39.87%, 44.91%, 39.76%, 43.09%, and 46.47% in the G4 (Cecal Slurry, LPS and E. coli along with untreated test formulation); G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15); G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se animals + untreated test formulation) groups, respectively, as compared to the disease control group (G2). Similarly, FDP level was decreased by 8.80%, 5.78%, and 11.39% in G6, G8, and G9 groups, respectively with reference to untreated test formulation (G4) group. Sepsis is associated with SIRS and induction of intravascular fibrin formation. Based on one of the clinical trials observations, reported that patients with SIRS and associated with sepsis the level of FDP is too high in comparison with the healthy individuals [43]. Overall, here the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly reduced the level of FDP, which could be beneficial in the SIRS and sepsis patients.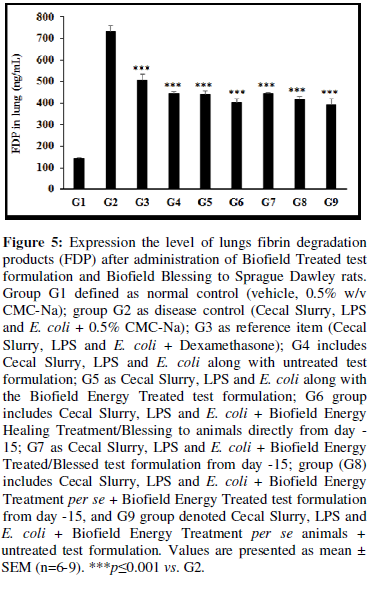
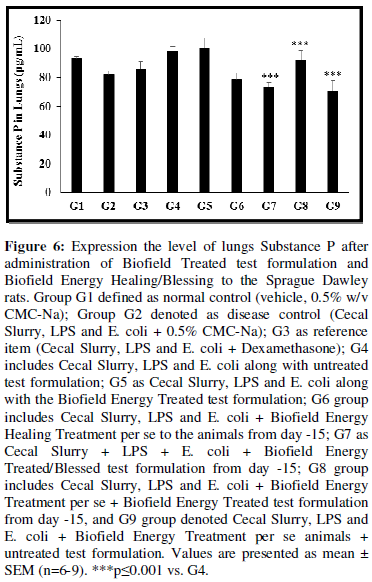
Estimation of Substance P
The level of lungs substance P was detected in all the experimental groups and the data are presented in Figure 6. The disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) group (G2) and positive control (Dexamethasone) treatment (G3) showed value of substance P as 82.23 ± 2.47 and 86.09 ± 4.96 pg/mL, respectively. The level of substance P was decreased by 4.12%, 10.80%, 12.35%, and 13.68% in the G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS and E. coli along with the Biofield Treated test formulation administered from day -15); G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated test formulation from day -15), and G9 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation) groups, respectively with reference to disease control group (G2). Additionally, substance P level was significantly (p≤0.001) decreased by 19.93%, 25.51%, and 27.92% in the G7, G8, and G9 groups, respectively as compared to the untreated test formulation (G4) group. According to Ang SF et al. (2011), reported that the expression of substance P has increased in inflammation/septic condition through the activation of the ERK-NF-κB pathway [44]. Overall, here the Biofield Energy Treated test formulation and Biofield Energy Treatment per se has significantly reduced the level of substance P, which could be beneficial for the management of systemic inflammation-related disorders.
Estimation of Inducible Nitric Oxide Synthase (iNOS)
The level of lungs inducible nitric oxide synthase (iNOS) was detected in all the experimental groups and the data are presented in Figure 7. The disease control (Cecal Slurry, LPS and E. coli + 0.5% CMC-Na) group (G2) showed value of iNOS as 27 ± 1 IU/mL, which was increased by 89% as compared with the normal control (G1, 14.28 ± 0.39 IU/mL). Further, the positive control (Dexamethasone) treatment (G3) showed decreased iNOS level by 10.37% i.e., 24.19 ± 0.92 IU/mL as compared to the G2 group. The level of iNOS was significantly decreased by 7.33%, 15.26% (p≤0.001), 39.26% (p≤0.001), 38.95% (p≤0.001), 47.63% (p≤0.001), and 59.78% (p≤0.001) in the G4 (Cecal Slurry, LPS and E. coli along with untreated test formulation); G5 (Cecal Slurry, LPS and E. coli along with the Biofield Energy Treated test formulation); G6 (Cecal Slurry, LPS and E. coli along with Biofield Energy Treatment per se to animals from day -15); G7 (Cecal Slurry, LPS and E. coli + Biofield Energy Treated test formulation from day -15); G8 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se + Biofield Energy Treated/Blessed test formulation from day -15), and group G9 (Cecal Slurry, LPS and E. coli + Biofield Energy Treatment per se animals + untreated test formulation), respectively with reference to disease control group (G2). Similarly, iNOS level was decreased by 8.55%, 34.45%, 34.11%, 43.49%, and 56.60% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the untreated test formulation (G4) group. More generation of NO (key endothelium-derived relaxing factor) due to influence of iNOS, which expressed due to overproduction of proinflammatory cytokines, is a major mechanism of endothelial dysfunction, and that are responsible for various abnormalities [45,46]. Overall, here the Biofield Energy Treated test formulation and Biofield Energy Treatment per se significantly reduced the level of iNOS, which could be beneficial for the management of inflammation-related disorders.
The present experiment includes four preventive maintenance groups viz. G6, G7, G8, and G9. The study outcomes showed the remarkable slowdown of inflammation-related symptoms and also reduced the chances of disease susceptibility. All-inclusive, it indicates that the Trivedi Effect® was found to be most effective and benefited to protect different kinds of diseases and also improve the overall health and quality of life.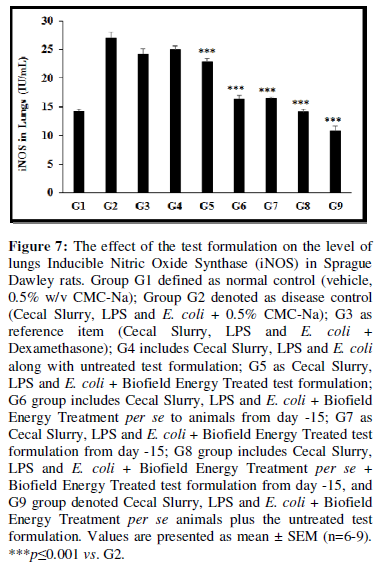
CONCLUSIONS
The level of MPO was significantly reduced by 51.44%, 71.69%, 55.79%, 55.16%, and 58.12% in the G5, G6, G7, G8, and G9 groups, respectively with reference to disease control (G2) group. MDA was significantly decreased by 52.71%, 56.54%, 67.35%, and 75.28% in G6, G7, G8, and G9 groups, respectively with reference to untreated test formulation (G4) group. Moreover, the level of MMP-9 was significantly reduced by 34.79%, 48.57%, 39.29%, and 41.25% in G5 to G9 groups, correspondingly with reference to untreated group (G4). Additionally, FDP was significantly decreased by 39.87%, 44.91%, 39.76%, 43.09%, and 46.47% in the G5, G6, G7, G8, and G9 groups, respectively as compared to the G2 group. Substance P was significantly decreased by 19.93%, 25.51%, and 27.92% in the G7, G8, and G9 groups, respectively with reference to G4 group. Further, the level of iNOS was significantly decreased by 15.26%, 39.26%, 38.95%, 47.63%, and 59.78% in the G6, G7, G8, and G9 groups, respectively as compared to the G2 group. Altogether, the Biofield Energy Treated test formulation and Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed fruitful results with respect to different antioxidants and inflammatory biomarkers in the preventive maintenance group, G6 as well as other preventive maintenance groups (G7, G8, and G9) in Cecal Slurry, LPS and E. coli-induced systemic inflammatory response syndrome (SIRS) model rat model study. It also helped to slowdown the inflammatory disease progression and disease-related complications. The study data showed that Biofield Energy Treated Test formulation and Biofield Energy Treatment per se could be one of the treatment approaches to prevent the manifestation of diseases. Thus, the Biofield Energy Treatment might act as a preventive maintenance therapy to maintain and improve the overall health and quality of life and simultaneously reduce the severity of acute/chronic diseases. The test formulation can also be used against rheumatoid arthritis (RA), fibromyalgia, aplastic anemia, Addison disease (AD), multiple sclerosis, myasthenia gravis, psoriasis, Crohn’s disease, ulcerative colitis, dermatitis, hepatitis, Parkinson’s, stroke, etc.
ACKNOWLEDGEMENTS
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
- Chakraborty RK, Burns B (2020) Systemic Inflammatory Response Syndrome. [Updated 2020 Apr 28]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan-. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK547669/
- Balk RA (2014) Systemic inflammatory response syndrome (SIRS): Where did it come from and is it still relevant today? Virulence 5(1): 20-26.
- Comstedt P, Storgaard M, Lassen AT (2009) The systemic inflammatory response syndrome (SIRS) in acutely hospitalized medical patients: A cohort study. Scand J Trauma Resusc Emerg Med 17: 67.
- Szekely Y, Arbel Y (2018) A review of interleukin-1 in heart disease: Where do we stand today? Cardiol Ther 7(1): 25-44.
- Yabluchanskiy A, Ma Y, Iyer RP, Hall ME, Lindsey ML (2013) Matrix metalloproteinase-9: Many shades of function in cardiovascular disease. Physiology (Bethesda) 28(6): 391-403.
- Salvemini D, Riley DP (2000) Nonpeptidyl mimetics of superoxide dismutase in clinical therapies for diseases. Cell Mol Life Sci 57(11): 1489-1492.
- Younus H (2018) Therapeutic potentials of superoxide dismutase. Int J Health Sci (Qassim) 12(3): 88-93.
- Sternberg EM (2006) Neural regulation of innate immunity: A coordinated nonspecific host response to pathogens. Nat Rev Immunol 6: 318-328.
- O'Connor TM, O'Connell J, O'Brien DI, Goode T, Bredin CP, et al. (2004) The role of substance P in inflammatory disease. J Cell Physiol 201: 167-180.
- Tsukahara Y, Morisaki T, Horita Y, Torisu M, Tanaka M (1998) Expression of inducible nitric oxide synthase in circulating neutrophils of the systemic inflammatory response syndrome and septic patients. World J Surg 22: 771-777.
- Rayman MP (2000) The importance of selenium to human health. Lancet 356: 233-241.
- Beard JL, Connor JR (2003) Iron status and neural functioning. Ann Rev Nutr 23: 41-58.
- Peres FF, Lima AC, Hallak JEC, Crippa JA, Silva RH, et al. (2018) Cannabidiol as a promising strategy to treat and prevent movement disorders? Front Pharmacol 9: 482.
- Nagarkatti P, Pandey R, Rieder SA, Hegde VL, Nagarkatti M (2009) Cannabinoids as novel anti-inflammatory drugs. Future Med Chem 1(7): 1333-1349.
- Kang S, Min H (2012) Ginseng, the 'Immunity Boost': The effects of Panax ginseng on immune system. J Ginseng Res 36(4): 354-368.
- Maizes V, Rakel D, Niemiec C (2009) Integrative medicine and patient-centered care. Explore (NY) 5(5): 277-289.
- Bischof M, Del Giudice E (2013) Communication and the emergence of collective behavior in living organisms: A quantum approach. Mol Biol Int 2013: 987549.
- Cassidy CM (2004) What does it mean to practice an energy medicine? J Altern Complement Med 10(1): 79-81.
- Barnes PM, Bloom B, Nahin RL (2008) Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report 12: 1-23.
- Fan KW (2005) National center for complementary and alternative medicine website. J Med Libr Assoc 93: 410-412.
- Wisneski L, Anderson L (2009) The Scientific Basis of Integrative Medicine. Boca Raton, FL: CRC Press 205.
- Trivedi MK, Tallapragada RM (2008) A transcendental to changing metal powder characteristics. Met Powder Rep 63: 22-28, 31.
- Trivedi MK, Nayak G, Patil S, Tallapragada RM, Latiyal O (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. Ind Eng Manage 4: 161.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica). J Food Nutr Sci 3: 245-250.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Charan S, et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment on Citrobacter braakii: A urinary pathogen. J Clin Med Genom 3: 129.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. J Antivir Antiretrovir 7: 083-088.
- Nayak G, Altekar N (2015) Effect of biofield treatment on plant growth and adaptation. J Environ Health Sci 1: 1-9.
- Branton A, Jana S (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in male Sprague Dawley rats. Int J Clin Deve Anat 3: 9-15.
- Branton A, Jana S (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in male Sprague Dawley rats. Am J Clin Experi Med 5: 138-144.
- Kinney JP, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. Am J Life Sci 5: 65-74.
- Singh J, Trivedi MK, Branton A, Trivedi D, Nayak G, et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. Am J Pharmacol Phytother 2: 1-10.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Plikerd WD, et al. (2017) A systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. Int J Bioorg Chem 2: 135-145.
- Trivedi MK, Patil S, Shettigar H, Mondal SC, Jana S (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. J Integr Oncol 4: 141.
- Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) Influence of biofield treated vitamin D3 on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. Int J Biomed Eng Clin Sci 4: 6-14.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). Int J Nutr Food Sci 7: 30-38.
- Loria V, Dato I, Graziani F, Biasucci LM (2008) Myeloperoxidase: A new biomarker of inflammation in ischemic heart disease and acute coronary syndromes. Mediators Inflamm 2008: 135625.
- Yasui K, Baba A (2006) Therapeutic potential of superoxide dismutase (SOD) for resolution of inflammation. Inflamm Res 55(9): 359-63.
- Salvemini D, Riley DP (2000) Nonpeptidyl mimetics of superoxide dismutase in clinical therapies for diseases. Cell Mol Life Sci 57(11): 1489-92.
- Bartsch H, Nair J (2006) Chronic inflammation and oxidative stress in the genesis and perpetuation of cancer: Role of lipid peroxidation, DNA damage, and repair. Langenbecks Arch Surg 391(5): 499-510.
- Gentile F, Arcaro A, Pizzimenti S (2017) DNA damage by lipid peroxidation products: Implications in cancer, inflammation and autoimmunity. AIMS Genet 4(2): 103-137.
- Halade GV, Jin YF, Lindsey ML (2013) Matrix metalloproteinase (MMP)-9: A proximal biomarker for cardiac remodeling and a distal biomarker for inflammation. Pharmacol Ther 139(1): 32-40.
- Lee AC, Trivedi K, Branton A, Trivedi D, Nayak G, et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). Int J Nutr Food Sci 7: 30-38.
- Toh JM, Ken-Dror G, Downey C, Abrams ST (2013) The clinical utility of fibrin-related biomarkers in sepsis. Blood Coagul Fibrinolysis 24(8): 839-843.
- Ang SF, Moochhala SM, MacAry PA, Bhatia M (2011) Hydrogen sulfide and neurogenic inflammation in polymicrobial sepsis: Involvement of substance P and ERK-NF-κB signaling. PLOS One 6(9): e24535.
- Tang EH, Vanhoutte PM (2010) Endothelial dysfunction: A strategic target in the treatment of hypertension? Pflugers Arch 459(6): 995-1004.
- Besedina A (2016) NO-synthase activity in patients with coronary heart disease associated with hypertension of different age groups. J Med Biochem 35(1): 43-49.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Pathology and Toxicology Research
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)









