Research Article
Intracranial Empyema: Experience of the Neurosurgery Department of Conakry University Hospital
3504
Views & Citations2504
Likes & Shares
Introduction: Intracranial empyema’s are the rare intracranial suppurations but serious and potentially fatal prognosis in the absence of adequate treatment. The objective of our study was to determine the epidemiological, clinical, paraclinical, therapeutic and evolutionary characteristics of these suppurations in our department.
Materials and methods: This were a descriptive retrospective study of 88 intracranial empyema’s files collected over a period of 5 years (January 2014-December 2018) in the archives of the neurosurgery department of Conakry University Hospital.
Results: The frequency was 43.1% for all intracranial suppurations. The mean age was 14.6 years (2-42 years) with a sex ratio of 1.8 (57 males/31 females). The entrance door was dominated by otorhinolaryngological infections, 76.5% and unknown in 19%. The clinical manifestations were dominated by fever (90%), intracranial hypertension (66%) and focal signs (45%). The site was subdural in 59 cases and extradural in 29 cases. Surgical treatment involved 91% of patients and 9% were treated with antibiotic therapy alone. The evolution was favorable in 68.1% of cases with a mortality rate of 13.6%.
Conclusion: Intracranial empyema’s are the medical and surgical emergency. The forms of otorhinolaryngology origin are more frequent. Despite the therapeutic difficulties, the prognosis remains acceptable in our study with 13.6% of deaths.
Keywords: Empyema, Subdural, Extradural, Antibiotic therapy, Neurosurgery
ABBREVIATIONS
ICS: Intracranial suppurations; ORL: Otorhinolaryngology
INTRODUCTION
Intracranial empyema’s are the suppurations collected extracerebral (extra or subdural) [1]. They are rare intracranial suppurations (ICS), but have a serious and potentially fatal prognosis in the absence of adequate treatment [2]. In developing countries of the South, particularly in Black Africa, empyema remains a current problem [3]. In terms of diagnosis, modern imaging techniques are still not available, and in terms of prognosis, the difficulties of treatment and the conjunction of complications inherent to a delay in diagnosis explain the seriousness of these suppurations [4]. We report our experience in order to evaluate this pathology from an epidemiological, clinical, paraclinical, therapeutic and evolutionary point of view in our department.
METHODS
This was a descriptive retrospective study of 88 intracranial empyema’s files collected over a 5-year period (January, 2014 – December, 2018) in the archives of the neurosurgery department of Conakry University Hospital. The diagnosis was made by CT scan. Follow-up was 6 to 12 months. The parameters studied were frequency, age, sex, clinic, imaging, biology, treatment and evolution. We excluded all incomplete files, all empyema’s of tuberculosis origin and all empyema’s associated with abscesses. Data were collected and analyzed using SPSS 21.0 software.
RESULTS
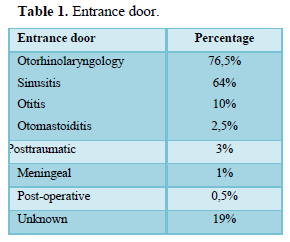
Out of 204 cases of ICS collected, intracranial empyema’s represented 88 cases, or 43.1%. The average age was 14.6 years (2 years-42 years) with a sex ratio of 1.8 (57 males/31 females). The entrance door was dominated by otorhinolaryngological infections, 76.5% and unknown in 19% (Table 1).
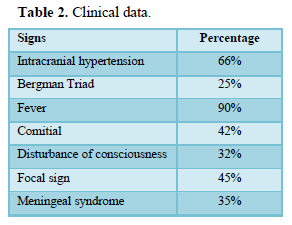
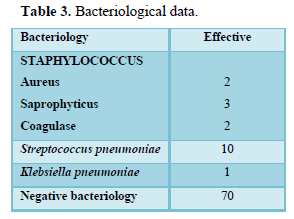
The time to diagnosis varied from 3 days to 3 months. Clinical data are summarized in Table 2. Biology showed hyperleukocytosis in 90%, C-reactive protein positive in 80%. The bacteriology was 80% negative, the germ was found in 18 cases (Table 3).



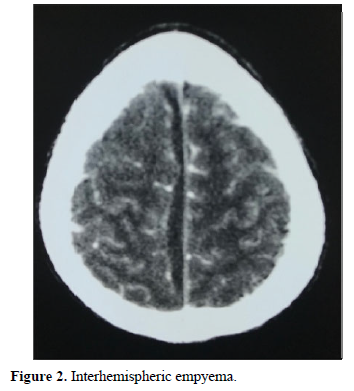
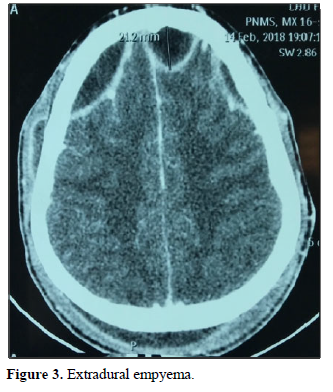
In terms of imaging, the empyema’s were subdural in 59 cases: hemispherical in 42 cases (Figure 1), interhemispheric in 16 cases (Figure 2) and one case of the tent of the cerebellum, and extra Dural 29 cases (Figure 3).
Therapeutically, a probabilistic tri-anti biotherapy, combining a 3rd generation cephalosporin (100 mg/kg weight), metronidazole (40 mg/kg weight) and combined in 54% with gentamycin (4 mg/kg weight) or thiamphenicol in 46% (50 mg/kg weight) was instituted for 3 weeks parenterally and continued orally for 4 to 8 weeks. It was then adapted to the results of the antibiogram. The treatment was 9% exclusive medical for the collections measuring less than 2 cm, in 91% of the cases the surgery was associated with a delay ranging from 24 h to 3 months. For the majority of cases, it was performed within the first 24 h with 74 cases of trepanation, 11 cases of flaps and 3 cases of craniectomies. It was justified on the basis of size and clinical tolerance criteria, in particular disorders of consciousness associated with a compressive collection. The entrance door was treated in 60% of cases. Anticonvulsant therapy (42%), corticosteroid therapy (13%) for intracranial hypertension associated with cerebral oedema and heparin therapy (3%) for cerebral thrombophlebitis were also associated.
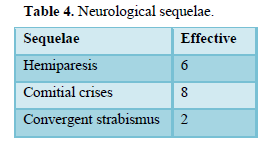
The evolution was marked by healing without sequelae in 60 cases (68.1%), healing with sequelae in 16 cases (18.1%), 6 cases (6.8%) of recurrence, requiring re-intervention and 12 cases (13.6%) of death. Table 4 summarizes the neurological sequelae.

DISCUSSION
The management and prognosis of empyema’s have evolved considerably in recent decades with the advent of CT scan in Guinea, allowing early diagnosis and a more precise and less aggressive treatment.
The frequency of this pathology is certainly underestimated in developing countries, especially in sub-Saharan Africa, due to under-equipment in medical imaging [5]. It was 43.1% in our series over a period of 5 years and 44.4% in Senegal over a period of 4 years [4].
The male predominance found here is classic regardless of the gateway and the type of ICS [4,6-8]. As for the age (mean: 14.6 years), it is consistent with the data in the literature [4,9-11] and is explained by the higher incidence of sinus and otomastoid infections at this time period of life [12], which remained predominant in our study with 76.5% of cases. The entrance door is unknown in 2 to 29% depending on the series [4,13] and in 19% in our series.
Clinically, the fever is inconstant [8,14,15] but present in 90% in our series ranging from 39 to 40◦ C. Intracranial hypertension varies from 69 to 100% of cases [16], 66% in our series. It is thought to be linked to the underlying cerebral oedema and thrombophlebitis of the upper sagittal sinus [16-18]. The signs of focusing are present in 75 to 100% of patients [10,13,19] against 45% in our series. The seizures vary from 22.2 to 44% of cases [18,20,21] and 42% in our series. The meningeal syndrome found in 14 to 75% of cases [10,15,18] is 35% in our series. Consciousness disorders represent 32% in our series against 63% in the Senegalese series [4]. The delay in diagnosis would explain these disorders of consciousness. The patients being admitted most often at a very advanced stage.
Staphylococcus and Streptococcus predominated in our series, which is similar to the series by Ouiminga [4] in Senegal. The pus is sterile in 17 to 67% of cases [8,13,17] against 80% in our series. Preoperative antibiotic therapy, conditioning of the sample in the event of anaerobic bacteria can be implicated [4].
The subdural site of empyema’s was predominant in our series (59 cases), which agrees with the data in the literature [7,22-24]. Extradural empyema is rarer, occurs in the majority of cases with the onset of osteitis or osteomyelitis of the vault of the skull. It occasionally complicates sinusitis or otomastoiditis [4].
The treatment is medical and surgical. The medical treatment is based on long-term antibiotic therapy with molecules diffusing well into the brain parenchyma [7,23-25]. The antibiotic treatment will be probabilistic from the outset, then secondarily adapted according to the germ detected and the antibiogram. If there is no germ, treatment will be empirical depending on the clinical course of the patient. The combination of a third-generation cephalosporin with metronidazole seems to us to be an interesting choice both via the venous and oral routes. Americans prefer the combination of a third-generation cephalosporin (ceftriaxone or cefotaxime) and thiamphenicol (1 g intravenous every 6 h) [1]. Intravenous treatment will be prescribed for 15 to 21 days and the relay orally for 15 days to 2 months [7,23,26]. In our practice, the protocol was 21 days parenterally and 4 to 8 weeks oral bi-antibiotic therapy. Corticosteroid therapy is indicated in life-threatening intracranial hypertension [19] and significant oedema, as was the case in 13% of our patients. Anticonvulsant treatment can be combined with antibiotic treatment throughout its duration [6,22,27], it was associated in 42% in our series. The entrance door must be systematically treated to avoid recurrence [4,20,27,28].
Surgically, single or multiple trepanations is less invasive and remains aesthetic but has certain drawbacks [30,24]: obstruction of the holes with thick pus or oedema; limited exploration of the brain; a significant risk of recurrence [18,19,29]. Drainage is performed for 3 to 5 days [16]. The bone opening can be enlarged, which would allow almost all of the collection to be removed and associated with abundant washing with physiological serum. The thick collections were thus easily evacuated without additional drainage because this material could have maintained the infection. This technique was performed in 74 of the patients in our series. Multiple trepanations would provide 77.8% cure [8]. Craniectomy would allow complete evacuation of the empyema leading to rapid clinical and radiological improvement [7]. This technique was effective for 3 patients in our series. However, it requires cranioplasty because of the aesthetic damage it causes, which limits its performance [10,26]. The placement of an implant in a weakened bone site whose therapeutic difficulty has been proven can, even after recovery, be a source of infection. For many authors, craniotomy using a bone flap remains the surgical technique of choice [11,14,26,28]. Performed in 11 of the patients in our series, it allows a wider exploration of the brain, looking for a possible residue of pus due to adhesions [6-28] and re-expansion of the brain in the event of severe cerebral oedema [33,37]. The survival rate is estimated to reach 77.8 to 91% when performed as a first-line treatment [8, 27] compared to 52% for trepanation [15,18,29]. It is reserved for inter-hemispherical, sub-temporal, posterior fossa empyema’s and multiple locations or when the collection is thick and viscous or in the event of the formation of a thick marginal capsule [30].
The course after treatment has improved considerably with the advent of antibiotics (80 to 100% mortality in the pre-antibiotic era) [7] but also in recent decades with the progress of medical imaging for diagnosis early. Nowadays, healing without sequelae is the rule in more than half of the cases: 44 to 78% according to the series [4, 6] and 68.1% in our series. Nevertheless, mortality remains significant, 13.6% in our series and 11% in the series in Senegal [4]. The possible neurological sequelae are variable: 18.1% in our series, 8 to 44% for the other series [4, 13, 18, and 27]. They are classified into “minor” sequelae (monoparesis, epilepsy, visual field deficit, minor behavioral disorders) and “major” sequelae (hemiplegia, severe behavioral disorders) [6]. The timing of the procedure is more important than the surgical technique itself. The late surgery observed in our experience is related to diagnostic difficulties. Early diagnosis, proper treatment and prompt surgery give the patient a better chance of recovery with few neurological complications. [15,28-30].
CONCLUSION
Intracranial empyema’s are the medical and surgical emergency. The forms of otorhinolaryngology origin are more frequent, and the difficulties of management from a diagnostic, therapeutic and economic point of view are the seriousness of this condition. Despite these hazards, the prognosis of our patients remains acceptable 13.6% of deaths. The combination of targeted antibiotic therapy and surgical treatment results in healing with fewer sequelae.
-
Leys D (2001) Brain abscesses and intracranial empyemas. Med Surg Encycl 17-485-A-10.
-
Emery E, Redondo A, Berthelot JL, Bouali I, Ouahes O, et al. (1999) Intracranial abscesses and empyemas: neurosurgical management. Ann Fr Anesth Reanim 18: 567-5
-
Loembe PM, Okomé-Kouakou M, Alliez B (1997) Suppurations collected intracranial in an African environment. Med Trop 5: 186-1
-
Ouiminga HAK, Thiam AB, Ndoye N, Fatigba H, Thioub M, et al. (2014) Intracranial empyemas: epidemiological, clinical, paraclinical and therapeutic aspects. Retrospective study of 100 observations. Neurosurg 60:299-
-
Soumaré M, Seydi M, Ndour CT, Fall N, Dieng Y, et al. (2005) Epidemiological, clinical and etiological profile of cerebral meningeal diseases observed at the infectious diseases clinic of the Fann University Hospital in Dakar. Med Mal Infect 35: 383-389.
-
Page C, Lehmann P, Jeanjean P, Strunski V, Legars D (2005) Abscesses and intracranial empyemas of O.R.L. Ann Otolaryngol Chir Cervicofac 122(3): 120-126.
-
Singh B, Van Dellen J, Ramjettan S, Maharaj TJ (1995) Sinogenic intracranial complications. J Laryngol Otol 109: 945-50.
-
Tewari MK, Sharma RR, Shuv VK, Lad SD (2004) Spectrum of intracranial subdural empyema’s in a series of 45 patients: Current surgical options and outcome. Neurol India 52(3): 346-349.
-
Hakim HE, Malik AC, Aronyk K, Ledi E, Bhargava R (2006) The prevalence of intracranial complications in pediatric frontal sinusitis. Int J Pediatr Otorhinolaryngol 70: 1383-1387.
-
Passeron H, Sidy Ka A, Diakhaté I, Imbert P (2010) Intracranial suppurations leading to otorhino-laryngologic entry in children in Senegal. Pediatr Arch 17: 132-140.
-
Kombogiorgas D, Seth R, Athwal R, Modha J, Singh J (2007) Suppurative intracranial complications of sinusitis in adolescence. Single institute experience and review of literature. Br J Neurosurg 21: 603-609.
-
Bernardini GL (2004) Diagnosis and management of brain abscess and subdural empyema. Curr Neurol Neurosci Rep 4: 448-4
-
Broalet E, N’dri Oka D, Eholie SP, Guillao-Lasme EB, Varlet G, et al. (2002) Abscesses and intracranial empyemas in children, observed in Abidjan. Afr J Neurol Sci 21(1): 38-
-
Gueye M, Badiane SB, Sakho Y, Koné S, Ba MC, et al. (1991) Brain abscesses and extra-cerebral empyemas. Dakar Med 36(1): 82-8
-
Yilmaz N, Kiymaz N, Yilmaz C, Bay A, Yuca SA, et al. (2006) Surgical treatment outcome of subdural empyema: a clinical study. Pediatr Neurosurg 42(5): 293-29
-
Gupta S, Vachhrajani S, Kulkarni AV, Taylor MD, Dirks P, et al. (2011) Neurosurgical management of extra axial central nervous system infections in children. J Neurosurg Pediatr 7(5): 4414
-
John TJ (2004) Subdural effusion or empyema in infants. Indian Pediatr 41(17): 968-9
-
Nathoo N, Narotam PK, Nadvi S, Van Dellen Jr (2011) Taming an old enemy: A profile of intracranial suppuration. World Neurosurg 77(3/4): 4844
-
Kojima A, Yamaguchi N, Okui S (2004) Supra and infratentorial subdural empyema secondary to septicemia in a patient with liver abscess. Neurol Med Chir (Tokyo) 44: 90-9
-
Ali A, Kurien M, Mathews SS, Mathew J (2005) Complications of acute infective rhinosinusitis: experience from a developing country. Singapore Med J 46(10): 54054
-
Djientcheu VP, Mouafo TF, Esiene A, Kamga YN, Nguefack S, et al. (2013) Intracranial suppurations in the African child: a severe but preventable complication. Childs Nerv Syst 29(1): 119-1
-
Albu S, Tomescu E, Bassam S, Merca Z (2011) Intracranial complications of sinusitis. Acta Otorhinolaryngologica Bel 55: 265-2
-
Korinek AM (1994) Brain abscesses and empyemas. Rev Prat 44: 2201-220
-
Younis RT, Lazar RH, Anand VK (2000) Intracranial complications of sinusitis: A 15-year review of 39 cases. Ear Nose Throat J 81: 636-63
-
Dolan RW, Chowdhury K (1995) Diagnosis and treatment of intracranial complications of paranasal sinus infections. J Oral Maxillofac Surg 53: 1080-108
-
Heran SN, Steinbok P, Cochrane DD (2003) Conservative neurosurgical management of intracranial epidural abscesses in children. Neurosurg 53(4): 893-89
-
Quraishi H, Zevallos JP (2006) Subdural empyema as a complication of sinusitis in the pediatric population. Int J Pediatr Otorhinolaryngol 70(9): 1581-158
-
Salunke PS, Malik V, Kovai P, Mukherjee K (2011) Falco tentorial subdural empyema: Analysis of 10 cases. Acta Neurochir (Wien) 153(1): 164-16
-
Mat Nayan SA, Mohd Haspani MS, Abd Latiff AZ, Abdullah JM, Abdullah S (2009) Two surgical methods used in 90 patients with intracranial subdural empyema. J Clin Neurosci 16(12): 1567-15
-
Edom KS, Kim TY (2011) Continuous subdural irrigation and drainage for intra- cranial subdural empyema in a 92-year-old woman. Mini Invas Neurosurg 54: 87-8
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Pathology and Toxicology Research
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)





