Research Article
The Application of Immunochromatographic Tests (ICT) in Rapid Screening of Brucellosis and Mers-Cov Infections in Camel
3833
Views & Citations2833
Likes & Shares
SUMMARY
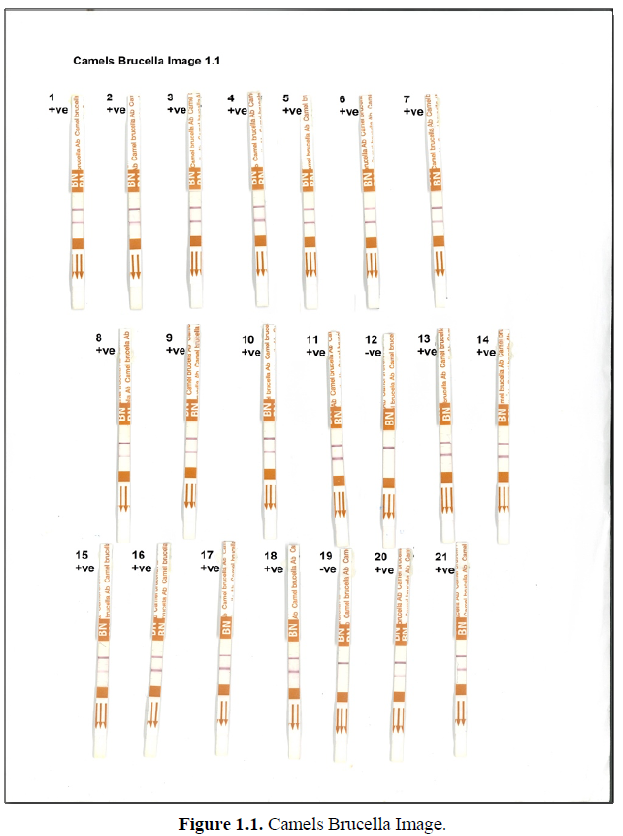
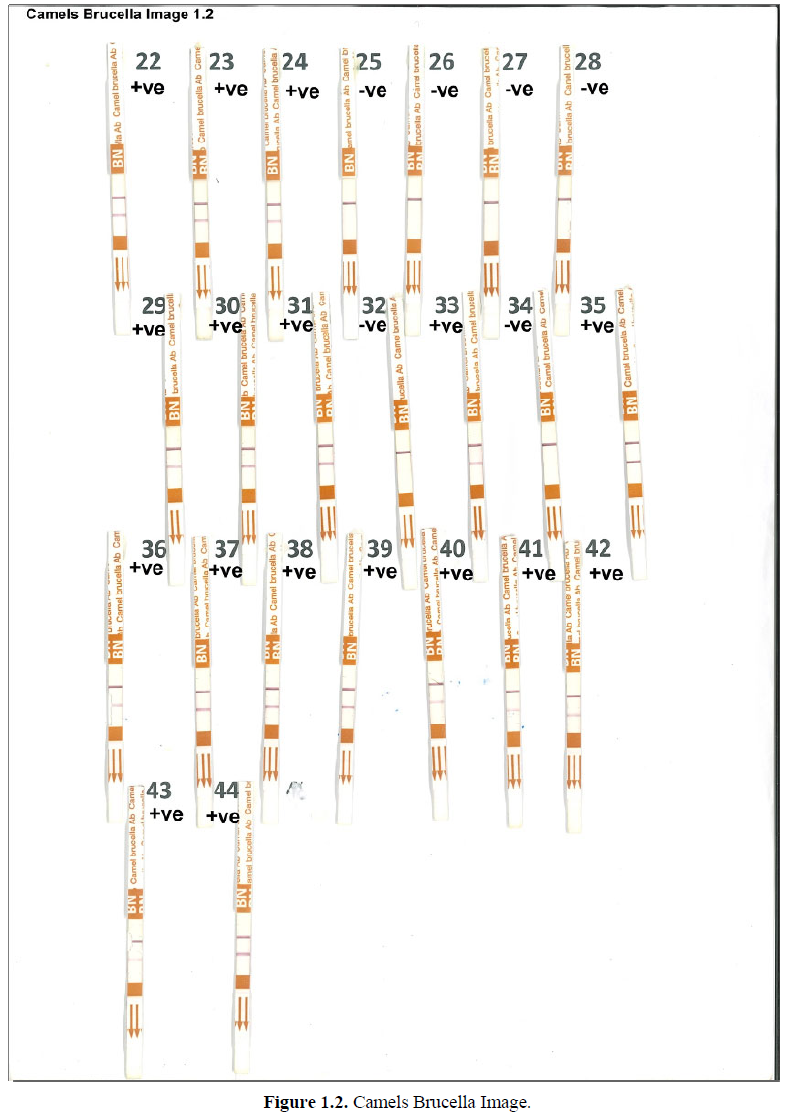
Brucellosis is a contagious disease caused by bacteria of the genus Brucella. Brucellosis in camel is caused mainly by two species of Brucella abortus and Brucella Melitensis. The disease can be transmitted between animals by direct contact, product of conception, body fluids, contaminated environment, contaminated water, feed and raw milk. In order to diagnosis camelid brucellosis, bacterial culture and isolation is the gold standard, however this method has a limited sensitivity, is laborious, time consuming and it is impractical to be used for a high number of samples. Therefore, serological test methods such as Rose Bengal (RBT), Complement Fixation test (CFT), Serum Agglutination test (SAT), Indirect and Competitive Immunoassays (i-Elisa, c-Elisa), and florescence Polarized assay (FPA), are considered the most practical methods to screen and confirm the diagnosis of this disease. More recently a new rapid immunochromatographic test (ICT) from Bionote, Korea, was used in detecting Brucella infection in dromedary camel. The test showed a sensitivity of 99.92% and specificity of 100% as mentioned by [1]. As it was illustrated in Figures 1.1 and 1.2, this Anigen rapid camel bucella Ab test was applied on 45 camel sera. The presence of two purple color bands inside the device window (C) and (T) indicated positive results.
The test was categorized negative when only device window (C) showed purple color. Out of 44 camel sera which were tested 36 were Burcella positive and 6 samples were Brucella negative. The major advantages of ICT camel rapid Ab test are that it can be used to detect camel brucellosis in serum, plasma, whole blood or milk, expanding the range of samples that can be used to diagnose brucellosis in camels. It is rapid and easy to perform without requiring special equipment.
Middle East respiratory syndrome coronavirus (Mers-CoV) is a newly identified human coronavirus associated with sever pulmonary syndrome and renal failure in infected patients. It is a zoonotic virus that has emerged in human in 2012 and caused severe respiratory illness with a mortality rate of 34.4 %. Since its appearance, Mers-Cov has been reported in 27 countries and the most of cases were in Saudi Arabia Mers-Cov outbreaks suggest that camels are a source of human infection. Nevertheless, the exact route of transmission from camels to humans remain unclear. The disease is diagnosed using RT-PCR as well as some serological tests primarily using S1- based Elisa kit to detect Mers-CoV (IgG) antibodies. Recently, Bionote, Korea has developed an immunochromatographic assay (ICT) for the rapid qualitative detection of Mers-Cov antigen in camel using nasal swabs. The assay based on the detection of Mers-Cov nucleocapsid protein in short time by highly selective monoclonal antibodies at room temperature. The sensitivity and specificity of the assay were found to be 93/9% and 100% respectively when compared with RT-PCR as mentioned by Song [2].
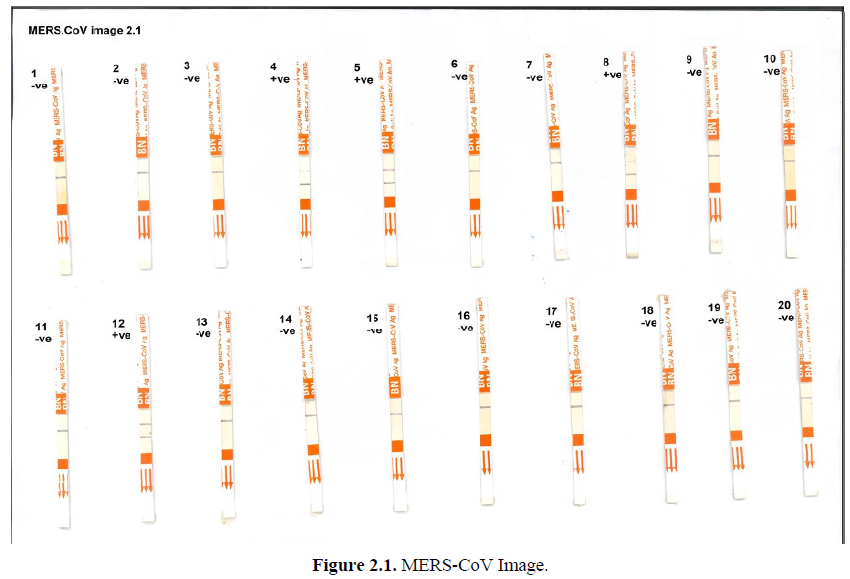
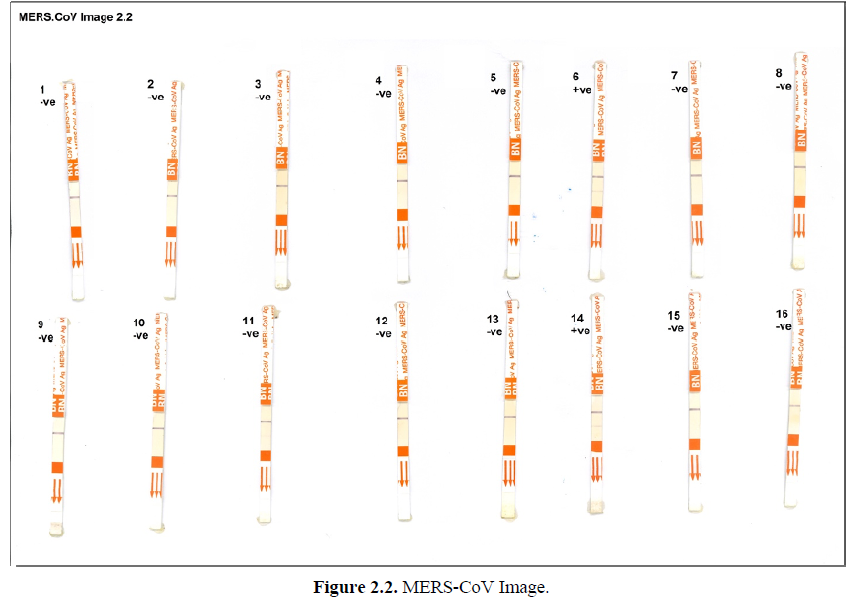
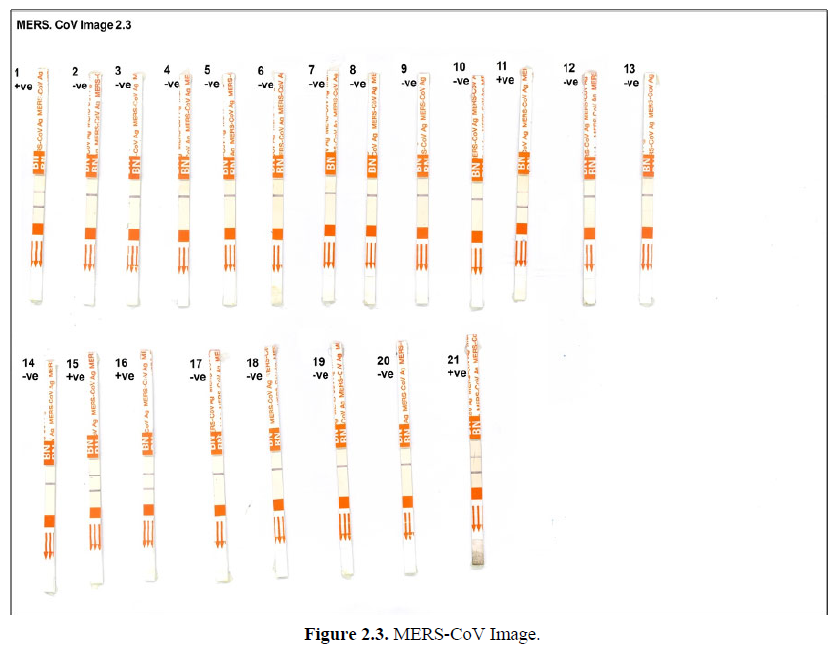
As it was illustrated in Figures 2.1-2.3, Mers-CoV antigen test was applied on nasal swabs. The presence of the purple band as the control (C) indicate absence of Mers-CoV antigen. While the presence of 2 purple bands at control (C) and test (T) indicates positive infection with Mers-CoV. Out of 57 nasal swabs from young camels (1-2) years old which were tested, eleven (11) samples were positive with Mers-CoV, while 46 were Mers-CoV negative.
Molecular tests are relatively expensive, not available in all laboratories and are mainly used for confirmatory purposes. For the purposes of screening of large number of animals in a short period of time, molecular tests are considered not particle; therefore, a rapid. Cheap, easy, sensitive and specific test is needed for diagnosis of Mers-Cov virus infection in camels.
On June 2016, Bionote Mers-CoV Ag test was officially registered in OIE by World Assembly of Delegates during the 84th general session held in Paris (France).
In accordance with the recommendation of OIE Biological standards Commission, the registered Mers-CoV Ag test is fit for the following purposes:
1-Detection of Mers-CoV infected herds (herd test) with acutely infected animals with high virus load.
2-When used as supplemental tests to estimate prevalence of infection to facilitate risk analysis, e.g., surveys, herd health schemes and disease control programmed.
The widespread Mers-Cov infection in camels might lead to a risk of future zoonotic transmission into people with direct contact with these infected camels thus indicates that camel pose s public health threat.





- Serhan WS, Khan RA, Gasim EF, Alketbi MS, De Massis F, et al. (2019) Performance of an Immunochromatographic Test (ICT) in Comparison to Some Commonly Used Serological Tests for the Diagnosis of Brucellosis in Dromedary Camels (Camelus dromedarius). Microorganisms 7(12): 591.
- Song D, Ha G, Serhan W, Eltahir Y, Yusof M, et al. (2015) Development and validation of a rapid immunochromatographic assay for detection of Middle East respiratory syndrome coronavirus antigen in dromedary camels. J Clin Microbiol 53(4): 1178-1182.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Pathology and Toxicology Research
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)







