Research Article
Compelling Evidence Implicating Human Papilloma Viral Infections of Placentas as the Primary Cause of Autism
4475
Views & Citations3475
Likes & Shares
ABSTRACT
The cause of autism spectrum disorder (ASD) is unknown. Identical twins share identical genetics but their concordance rate (both have it) is only 88% revealing the primary cause of ASD is environmental. Different prenatal environments exist when identical twins have separate placentas (~30% of the time). Scientists know inclusions in placentas are predictive of ASD and that the human papillomavirus (HPV) can infect the trophoblast cells of placentas and then transmit to the fetus where HPV infects the epithelial cells of the brain’s choroid plexus. HPV is known to cause epigenetic changes, deletions, and duplications of genes, which cause excessive increases in extra-axial cerebral spinal fluid seen by MRI in the brains of 6- and 12-month-old infants later diagnosed at 2 years. with ASD. Scientists also know male placentas implant close to the cervix (low-lying) three times more often than female placentas, which explains why the ASD ratio of boys to girls is ~3:1. Moreover, the HPV vaccination program that began in 2007 in Australia might be why the ASD incidence in the 0-4 years. age group did not increase from 2010 to 2015. This paper presents compelling evidence that placental HPV infections are the primary cause of ASD.

Keywords: Autism spectrum disorder, Placental infections, Choroid plexus, Human papillomavirus, Placental abnormality, Prenatal environmental exposure
ABBREVIATIONS
ASD: Autism Spectrum Disorder; CSF: Cerebral Spinal Fluid; HPV: Human Papillomavirus; PCR: Polymerase Chain Reaction
INTRODUCTION
Autism spectrum disorder (ASD) comprises a wide range of neurological developmental conditions characterized by deficits in social interactions and communication skills. The cause of ASD is unknown so that everything from genetics (including somatic mutations, polymorphisms, copy number variants, and epigenetic changes) to environmental exposures (chemicals, medications, drugs, cytokines, low vitamin D levels, vaccinations, etc.) and combinations of both have been investigated in a desperate attempt to thwart the alarming rate increase. Although we might explain about half of this alarming rate increase by enhanced public awareness and changes in diagnostic criteria [1], the rest of the rate increases in ASD incidences around the world over the past few decades are real, as shown by the CHARGE study [2]. In 2017, the rate varied in developed countries around the world from 1 in 27 in Hong Kong to 1 in 3,333 in Poland [3], and some argued the United States (U.S.) was as high as 1 in 45 [4]. ASD is now recognized as a pandemic disease that scientists once thought was genetic, but the fact that it has been increasing in recent decades at alarming rates in all races around the world suggests it is primarily an environmental factor.
Identical (monozygotic) twin studies supply the best evidence some environmental factor causes ASD. Identical twins, who share identical genetics, have less than a 100% concordance rate (both have it). If ASD is a genetic disorder, we expect pairwise concordance rates of 100%, but we only observe an 88% pairwise concordance rate between identical twins and a 31% pairwise concordance rate between non-identical twins from a large twin study involving 277 twin pairs [5]. Surprizingly, a recent quantitative analysis of 366 idenitcal twin pairs revealed that although the proband concordance rate for ASD was high (~96%) the similarity of shared sympotms between identical twins was low and so it was concluded that some non-shared environmental factor during early development is responsible for the broad range of genetic and behavoral differences observed between identical twins [6]. This combined twin data supplies critical clues in the etiology; children do not inherit (genetic origin) ASD because the concordance rates between identical twins are not 100%, and even when the identical twins are concordant the degree of ASD symptoms differs broadly. These twin observations also clarify the fact that environmental soluble factors like chemical exposures, medications, drugs, vaccines, alcohol, cytokines (e.g., IL-17a), any blood-born substances or infections, or even lower prenatal levels of vitamin D [7] cannot be the primary cause of ASD. For if any environmental soluble factor was primarily responsible for causing ASD, then all twins - identical or not - would share that exposure and would have a 100% concordance rate and would also have the same degree of symptoms, but we do not observe this. If a blood-borne virus like HIV [8], Herpes simplex virus [9], cytomegalovirus, or Epstein Barr Virus [10] infects the mother and is the primary cause of ASD, then all twins would have a 100% concordance rate. And although some soluble factors like infections in utero have been confirmed to be associated with a significant increased risk for ASD and occur in all twins causing both to have ASD and to be concordant, these types of infections cannot be the primary cause of ASD because they do not explain the discordant rates between identical twins [11]. To know the primary infectious agent that causes ASD, one must explain how the concordance rate of ASD can be less than 100% for identical twins who have identical DNA and are assumed to share the same prenatal environment. But what happens if we remove that assumption?
To investigate what might cause ASD, we will:
- examine the prenatal environments (placentas) of identical and non-identical twins
- compare the prevalence of cervical HPV infection, ASD, and placenta previa worldwide
- investigate how HPV can infect the brain and what it might do
- determine if any biochemical and epigenetic fingerprints of HPV are present in ASD children
- consider ways to prevent ASD, predict it clinically, and test to see if this hypothesis is correct
PRENATAL ENVIRONMENT - IDENTICAL VERSUS NON-IDENTICAL TWINS
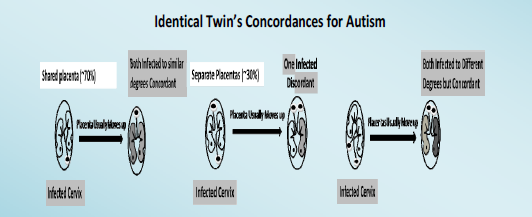
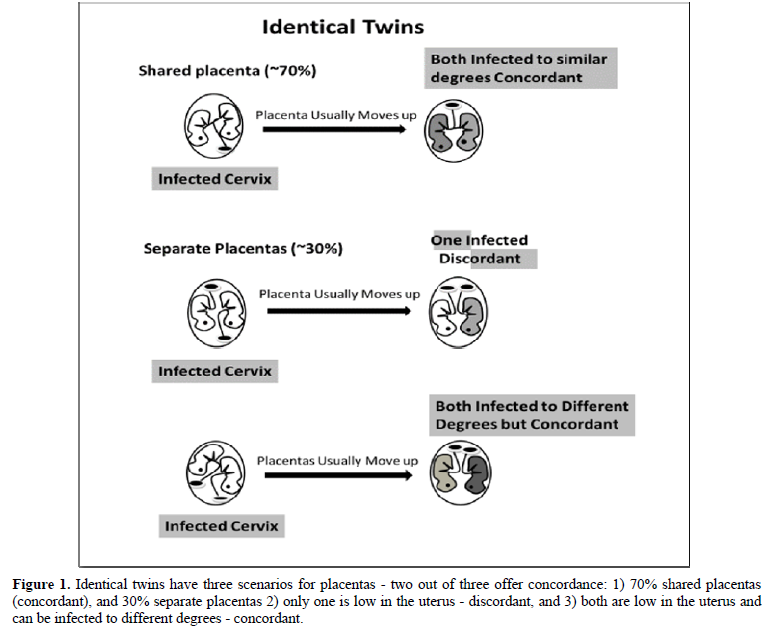
We should ask what difference exists between identical twin’s prenatal environments that produces a concordance rate lower than 100%. This is the most important question concerning the etiology of ASD because the answer explains why different concordance rates exist between identical twins that share identical genetics yet do not always share ASD at all or to the same degree [6]. For the following discussion cells of the skin, mucosal cells of the cervix and oral cavity (ref), for identical twins. ASD and Examination of the prenatal environment reveals identical twins only share the same placenta about 70% of the time (monochorionic), while non-identical twins never share the same placenta (dichorionic) [12].
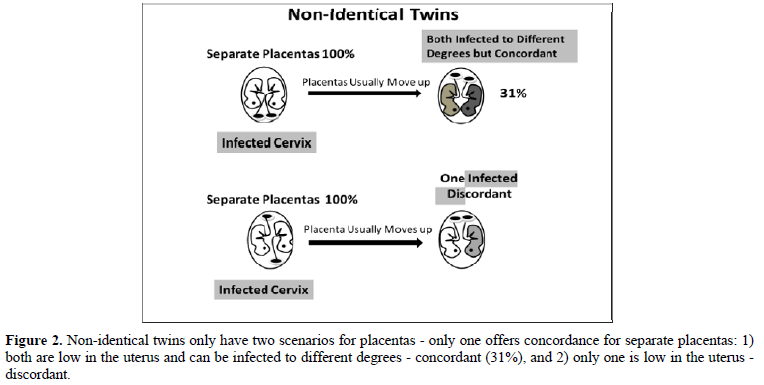
If the placenta implants close to a contaminated cervix, the mother can vertically transmit that infection to her fetus [13,14]. If placentas implant near the cervix (low-lying), they usually move upward in the uterus during the second or third trimester; about 90% of the low-lying placentas cleared the cervix by 32 weeks and about 96% of the low-lying placentas cleared the cervix by 36 weeks [15] avoiding placenta previa at birth [16]. If a cervical infection causes ASD, identical twins will be concordant if either the shared placenta (70%) is near the infected cervix (and both become infected to the same degree; see top of Figure 1) or both non-shared placentas (30%) are near the infected cervix (and one twin might become more infected than the other; bottom of Figure 1) because they will be discordant for ASD if only one placenta is near the infected cervix (middle of Figure 1). For non-identical twins to be concordant for ASD (Figure 2), both placentas must be close to the infected cervix (top of Figure 2), for if only one placenta is close to the infected cervix, they will be discordant (bottom of Figure 2). We can estimate the probability of both identical twin placentas being close to the cervix based on the non-identical twin concordance rate of 31% [5]. To get the total concordance rate estimate for identical twins, we multiply 31% (probability of both placentas being near the cervix) by 30% (non-shared placentas) and get about 10% that we add to 70% (shared placentas being near the cervix) for an estimated 80% pairwise concordance rate, which is within 10% of the observed 88% concordance rate for identical twins [5]. Note that some studies found lower concordance rates for identical twins. Compliant with this hypothesis is the fact that males tend to have low-lying placentas three times more often than females, as shown by their placenta previa rates [17], which matches the ratio of males to females with ASD (~3:1) [4,18].
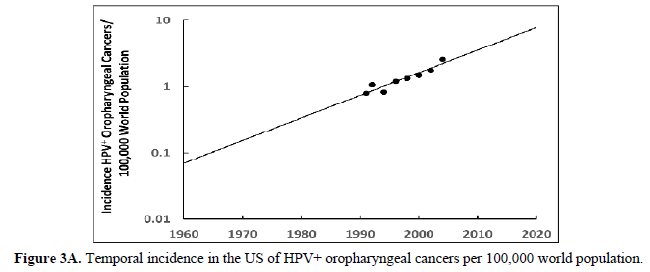
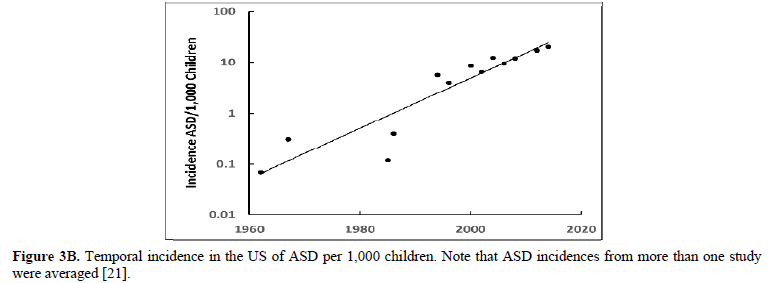
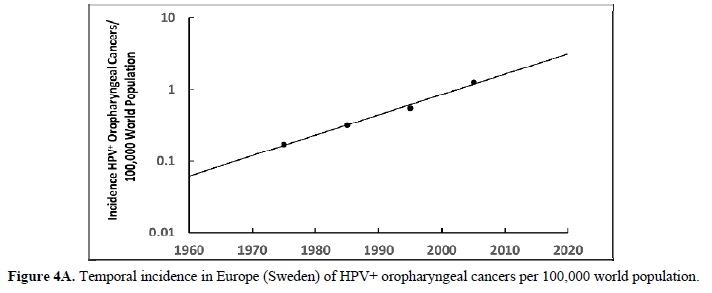
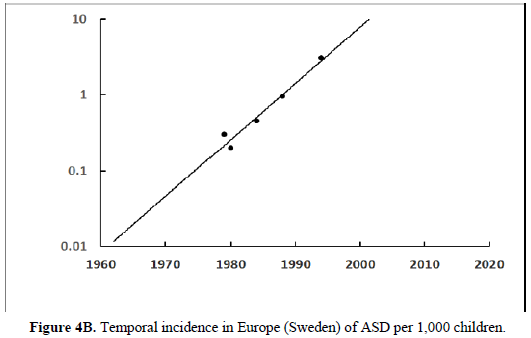
The human papillomavirus (HPV) is the most prevalent cervical infection around the world [19]. The exponential increase in HPV+ oropharyngeal cancers in the US [20] (Figure 3A) reflects the exponential increase in the incidence of HPV infection and during a similar timeframe there was also an exponential increase in ASD in the US (Figure 3B) [21]. We observe this phenomenon in developed countries worldwide as displayed in Europe (Sweden) where the HPV+ oropharyngeal cancers reflect the exponential increase in HPV infection (Figure 4A) and during a similar timeframe there was also an exponential rise in ASD (Figure 4B). Besides the oral cavity and the cervix, clinicians find HPV in the trophoblast cells of placentas [22,23] that can transmit HPV to the fetus [13,14]. We know that trophoblast inclusions are predictive of ASD [24] and that placental abnormalities and preterm births are major risk factors for ASD [25]. Trophoblast inclusions result from abnormal infoldings of the trophoblast bilayer, which can be from an increased number of cytotrophoblasts. Although clinicians have never tested for HPV in these ASD placentas that have trophoblast inclusions, we know HPV can cause placental abnormalities, preterm births, low birth weights, and spontaneous abortions [26,27]. The severity of ASD might depend on the strain(s) of HPV (e.g., -16, -18, -52, etc.), the viral load (how close the placenta imbeds near the cervix), the timing of infection (first trimester or longer), or all these parameters combined that might explain the spectrum of autistic symptoms.






Worldwide Prevalence of Cervical HPV Infection, ASD, and Placenta Previa
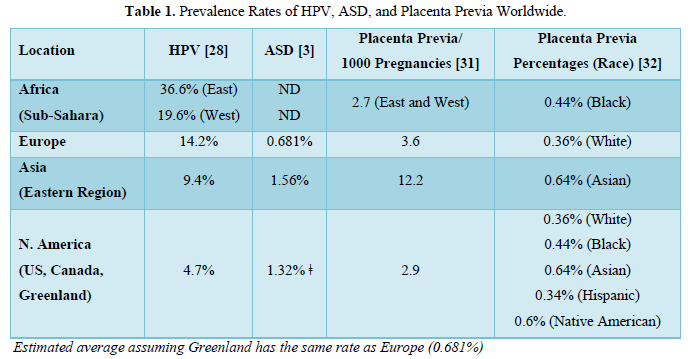
The average prevalence of cervical HPV infection in 2010 was determined to be about 11.7% worldwide from a sampling of about one million women of all ages [28]. The prevalence varied by age, continent, and region. The older the women, the lower the HPV prevalence. More than 65% of the women tested were 45 yr. or older and that age group had the lowest prevalence for HPV (4.2-7.5%). The younger women primarily of child-bearing ages had the highest prevalence of HPV that increased with decreasing age; the prevalence of HPV infection was 9.1% in the 34-44 yr. age group, 13.9% in the 25-34 age group, and 24% in the under 25 yr. age group. The continent with the highest HPV prevalence was Africa (21.1%) followed by Europe (14.2%), the Americas (11.5%; includes Canada, US, Greenland, Central and South Americas, and the Caribbean islands), and Asia (9.4%); that study excluded Australia because they have an ongoing HPV vaccination program since 2007 (Table 1). In Africa, the regions with the highest prevalence were in the eastern (33.6%) and western (19.6%) Sub-Saharan countries. In Europe, the regions with the highest prevalence of HPV were in the eastern (21.4%) and northern countries (10%). In the Americas, the regions with the highest prevalence of HPV were in the Caribbean (35.4%) and South (15.3%) and Central (15.3%) Americas and the region with the lowest prevalence was in North America (4.7% averaged for the U.S., Canada, and Greenland). In Asia, the regions with the highest prevalence of HPV were in the southeast (14%) and east (10.7%; Mongolia, South Korea, Japan, and China). The order of HPV prevalence is Africa (21.1%)> Europe (14.2%) > Americas (11.5%) [N. America (4.7%)] >Asia (9.4%).
The order of HPV prevalence around the world in 2010 does not match the order of ASD prevalence around the world in 2017 (3; note that diagnosis of ASD in children is usually by age 8). The ASD rates in Africa were not determined, but in Europe the average rate was about 0.681%. The ASD rates were not determined in South or Central America or Greenland but were 1 in 45 (2.22%) in the US and 1 in 94 (1.06%) in Canada. Unfortunately, we cannot calculate an average ASD rate in North America because it was not determined in Greenland but if we assume that rate was like Europe’s rate (0.681%) then the average rate is about 1.32%. The ASD rates were not determined in the west, south, or southeast of Asia but were determined in eastern Asia and were 1 in 38 (2.63%) in South Korea, 1 in 55 (1.82%) in Japan, and 1 in 435 (0.23%) in China for an average of 1.56%. The order for ASD is Asia (~1.56%) > N. America (~1.32%) > Europe (~0.681%, excluding southern regions); that study did not assess Africa and Australia.
The order of HPV prevalence is the opposite order of ASD prevalence in similar regions of the world. However, cervical HPV infection is not the sole predictor of ASD, because the placenta cannot get HPV unless it is implanted low in the uterus near the infected cervix. So, all mothers could be infected with HPV and not have a child with ASD if the placenta is implanted high in the uterus. Placenta previa rates reflect the rate of placentas implanting low in the uterus whether they stay there during the entire pregnancy or not. As high as 30% of placentas are low-lying in the uterus prior to 24 weeks gestation [29] 90% of which moved higher up in the uterus prior to delivery avoiding placenta previa [30]. The placenta previa rates were highest in Asia (12.2 per 1000 pregnancies) but lower in Europe (3.6 per 1000 pregnancies) and North America (2.9 per 1000 pregnancies) and were the lowest in Sub-Saharan Africa (2.7 per 1000 pregnancies) [31]. Because the region of the world can either reflect contributions towards placenta previa rates from something in the environment (e.g., food, water, chemical exposures) or from genetics (race/ethnicity), the study performed in one location in northern California involving different races shows genetics plays the biggest role in placenta previa rates [32]: Asians (0.64%) > Native Americans (0.6%) > African Americans (0.44%) > Caucasians (0.36%) > Hispanics (0.34%). The placenta previa rates are more predictive of ASD than HPV prevalence as Asians have the highest placenta previa rates of all the races and some regions of Asia have the highest ASD rates in the world like Hong Kong (1 in 27 or 3.7%), South Korea (1 in 38 or 2.63%), and Japan (1 in 55 or 1.82%).
The prevalence of HPV does not determine the prevalence of ASD because the placenta must be located near the cervix to become infected. Thus, the placenta previa rates are better predictors of ASD than HPV prevalence. Furthermore, because HPV prevalence is age dependent and the averages that are presented for the continents and regions of the world are primarily for older women (65% ≥45 yrs.), it is not a reliable way to predict ASD if HPV is responsible for causing it. If HPV causes ASD, neither HPV nor placenta previa prevenances can predict the ASD rate because it is a combination of both that varies in the world by region, race, and age of the mother.

Can HPV infect the brain and what can it do?
Scientists find HPV in neurons [33] and in the post-mortem brains of children that had focal cortical dysplasia type IIB (epilepsy and seizures) [34,35] a condition also associated with ASD [36]. Among the five HPV genera, alpha, beta, gamma, mu, and nu [37], the beta and gamma genera infect epithelial cells and can infect the epithelial cells of the choroid plexus [38], a centrally located lining inside the brain responsible for producing cerebral spinal fluid (CSF). The epithelial cells of the choroid plexus have the sodium-driven chloride bicarbonate exchanger, SLC4A10 gene product, required for CSF production [39]. SCL4A10 secretes CSF by unidirectionally transporting sodium, chlorine, and bicarbonate from the blood to the ventricles of the brain. Disruption of SLC4A10 augments neuronal excitability, modulates synaptic short-term plasticity [40], and in some cases might be associated with mental retardation and complex partial epilepsy [41]. Notably, increases in extra-axial CSF at 6 and 12 months of age observed by MRI predicted which infants developed ASD at 2 yrs. of age, and increasing amounts of CSF correlated with increasing symptoms of ASD [42]. Most importantly, integration of HPV into the genome [43] results in duplications and deletions of genes and an increased copy number of the SLC4A10 gene is associated with ASD [44]. We obtain more evidence HPV causes problems in the brain from the papilloma’s [45] and tumors [46] found in the choroid plexus of children less than 2 yrs. old. Transgenic mice expressing HPV-16’s E6 and E7 oncogene proteins have tumors in the choroid plexus [38]. And a hallmark of ASD is neuroinflammation caused by activation of the neuroglial cells [47] to remove an infection, as they are the first line of defense (innate immune response) in the brain [48]. In addition, HPV might also infect the gut because the gut has a single layer of epithelial cells like the choroid plexus with similar functions and immune responses [49] and most ASD children (~75%) have gastrointestinal diseases, including inflammatory bowel disease [50].
Do biochemical and epigenetic fingerprints of HPV exist in ASD children?
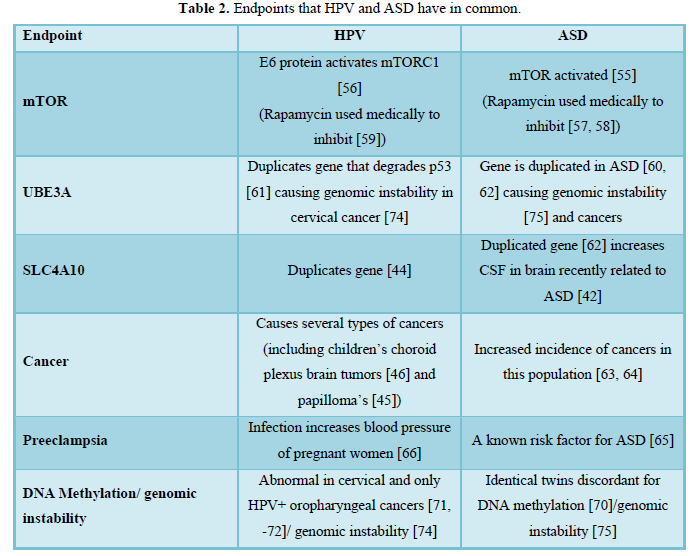
If HPV were involved in both ASD and cancer, we would expect to find biochemical similarities between ASD [51] and cancer [52], and in fact, many biochemical similarities exist between ASD and cancer [53] (Table 2). First, ASD and cancer both have disruptions in the PI3K-Akt-mTOR signaling pathway. The mTOR serine/threonine kinase is involved in brain development mediating signaling pathways essential for glial and neuronal differentiation and maintaining the stemness of neural stem cells. Problems with mTORC1 signaling are not only associated with ASD, but are also associated with pediatric brain tumors, seizures, learning disabilities, and mental retardation [54]. Children with ASD have activated mTOR signaling through PTEN [55] and HPV’s E6 protein activates mTORC1 signaling [56]. Rapamycin inhibits the mTORC1 pathway and clinicians use it to treat both ASD [57, 58] and cancer [59]. Second, the UBE3A gene that encodes the ubiquitin E3 ligase, E6-AP that degrades p53 – “the Guardian of the Genome” – is associated with ASD [60] and HPV [61]. Besides SLC4A10, UBE3A is another consistently duplicated gene in ASD [62]. Third, if HPV is the cause of ASD, we would expect to see significantly higher incidences of cancer in this population and we do [63, 64]. Fourth, ASD is associated with preeclampsia, or high blood pressure in pregnant women [65] and HPV infection is associated with an almost 2-fold increase in the risk for developing preeclampsia [66]. Finally, lower vitamin D3 levels are associated with all ASD children and are also associated only with the discordant identical twin who had ASD even though both twins got similar sun exposures [67]. HPV probably causes low vitamin D levels associated with ASD children by increasing the production of the enzyme alpha-N-acetylgalactosaminidase [68] that cleaves the entire sugar moiety off the vitamin D binding protein rendering it useless for binding to and transporting vitamin D into the bloodstream. Moreover, enzymatic conversion of the vitamin D binding protein to the macrophage-activating factor can no longer occur because the sugar moiety is missing resulting in suppression of the innate immune response. Additionally, because T cells require vitamin D for activation and antigen receptor signaling [69], low levels cause suppression of the adaptive immune response. HPV’s strategy of suppressing both the innate and adaptive immune responses allow it to escape immune surveillance and thrive in the body.
Epigenetic events can make diseases like ASD appear to be of genetic origin when they are not. For example, identical twins discordant for ASD are also discordant for DNA methylation [70] which is also abnormal in cervical cancer [71] and only in HPV positive – not HPV negative – oropharyngeal cancers [72]. Hypomethylation and hypermethylation of DNA promoters either decrease or increase production of gene products. HPV can integrate into the host genome at over 190 sites [73] and can disrupt multiple biochemical pathways by duplicating or deleting sections of the DNA causing genomic instability in cervical cancer (74), which also occurs in ASD [75]. Moreover, about 5% of the point mutations detected in ASD might be from methylation of cytosines in CpHpG sites (where H = A, C or T) that deaminated resulting in mutations [76]. Adding fuel to the genetic argument is the higher occurrence of ASD between siblings. However, this might be because over half the infected women cannot clear HPV for 2 years [77], which might also explain why one year of spacing between pregnancies is three times riskier for having a second child with ASD than 3 years of spacing between pregnancies [78]. If ASD were a highly inheritable disease, spacing time between births would not matter. Besides short spacing times (6 yrs.) between births also appear to increase the risk for ASD [79], which might be from reinfection or reactivation of HPV when the mother becomes immunosuppressed [80]. Immunosuppression can occur in a variety of ways and vitamin D3 deficiency (and insufficiency) is one way to reactivate HPV [81]. Note that gestational vitamin D deficiency [7] and either neonatal or combined maternal and neonatal insufficient levels of vitamin D [82] are associated with an increased risk for ASD. Thus, viral integration into the host’s genome resulting in duplications and deletions of genes, epigenetic events, and spacing times between sibling’s births (viral clearance) can make ASD appear to be of genetic origin when it is not.

PREVENTION, CLINICAL IMPLICATIONS, AND HYPOTHESIS TESTING
Prevention of ASD is the best course of action, as treatment modalities might not completely restore all brain functions. The best strategy to eradicate ASD is to give the HPV vaccine series to everyone over 1.5 yrs. of age (when the immune system is competent). Women of all ages should get the HPV vaccination series prior to and even during pregnancy, which doctors found safe [83], because they can supply passive immunity to the neonate and infant. Besides the general Australian population getting vaccinated against HPV, the free school quadrivalent HPV vaccination program for girls (ages 12-13 yr.) that began in 2007 (extended to boys in 2013), started an ongoing human population study which should, if this hypothesis is correct, produce a decline in ASD in that country (1 in 150 ASD in 2015) [18]. Vaccinated girls in 2007 aged 12-13 yr. and older were of child-bearing age in 2015 (20-21 yr. and older) so that the May and Williams [18] study included their children along with children born to older vaccinated mothers. As a result, Australia was the only country in the world that did not have an increase in ASD from 2010 to 2015 for the 0-4 yr. age group [18]. The ASD rate probably would have declined in that age group if the Australians had vaccinated everyone back in 2007. Concomitantly, people should raise their vitamin D3 to sufficient blood levels (≥50 nmol/L) so that the innate and adaptive immune responses can control or eliminate the virus while improving overall brain health [84].
Clinically, several risk factors exist that might potentially predict if a child will display ASD. Prenatal risk factors predictive of ASD include preeclampsia [65] and possibly low-lying placentas along with an HPV infected cervix (this paper). A perinatal risk factor predictive of ASD is inclusions in the placenta [24]. An infant risk factor predictive of ASD is increased extra-axial CSF at 6 or 12 months of age using MRI [42]. Testing for antibodies against HPV in the blood will not be a reliable way to predict ASD, as the child might be HPV+ but not have ASD because they either became infected perinatally during vaginal birth or as an infant by their infected parent kissing them on the mouth (or cleaning their pacifier with their mouth). Note that cervical Papanicolaou (Pap) smears only detect cancerous cells and not the presence of HPV, so these tests should also include a broad-spectrum HPV test along with routine Pap smears, especially for women who are trying to get pregnant or who already are pregnant.
Three ways to quickly test this hypothesis:
- Use immunohistochemistry, or another method like polymerase chain reaction (PCR) (85, 86) to assess if HPV is present in placentas with inclusions found to be predictive of ASD [24]
- Follow-up on the children who got HPV from their mothers to evaluate the ones who later developed ASD in vertical transmission studies like the ones performed by Smith [13] and Heller [14].
Screen the post-mortem brains of children with and without ASD immunohistochemically, or otherwise (e.g., PCR), for the presence of HPV, especially in the choroid plexus.
-
Hansen SN, Schendel DE, Parner ET (2015) Explaining the increase in the prevalence of autism spectrum disorders: The proportion attributable to changes in reporting practices. JAMA Pediatr 169(1): 56-62.
-
Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, et al. The CHARGE study: An epidemiologic investigation of genetic and environmental factors contributing to Environ Health Perspect 114(7): 1119-1125.
-
Xu G, Strathearn L, Liu B, Bao W (2018) Prevalence of AutismSpectrum Disorder Among US Children and Adolescents, 2014-2016. JAMA. 319(1): 81-82. Erratum in: Error in Source Data for Prevalence of Autism Spectrum Disorder. JAMA 319(5): 505.
-
Rosenberg RE, Law JK, Yenokyan G, Mc Gready J, Kaufmann WE, et al. (2009) Characteristics and concordance of autism spectrum disorders among 277 twin pairs. Arch Pediatr Adolesc Med 163(10): 907-914.
-
Castelbaum L, Sylvester CM, Zhang Y, Yu Q, Constantino JN (2020) On the Nature of Monozygotic Twin Concordance and Discordance for Autistic Trait Severity: A Quantitative Analysis. Behav Genet 50(4): 263-272.
-
Vinkhuyzen AAE, Eyles DW, Burne THJ, Blanken LME, Kruithof CJ, et (2018) Gestational vitamin D deficiency and autism-related traits: The Generation R Study. Mol Psychiatry 23(2): 240-246.
-
Musetti L, Albizzati A, Grioni A, Rossetti M, Saccani M, et al. (1993) Autistic disorder associated with congenital HIV infection. Eur Child Adolesc Psychiatry 2(4): 221-225.
-
Mahic M, Mjaaland S, Bøvelstad HM, Gunnes N, Susser E, et al. (2017) Maternal Immunoreactivity to Herpes Simplex Virus 2 and Risk of Autism Spectrum Disorder in Male Offspring. M Sphere. 2(1): e00016-17.
-
Valayi S, Eftekharian MM, Taheri M, Alikhani MY (2017) Evaluation of antibodies to cytomegalovirus and Epstein-Barr virus in patients with autism spectrum disorder. Hum Antibodies 26(3): 165-169.
-
Al-Haddad BJS, Jacobsson B, Chabra S, Modzelewska D, Olson EM, et al. (2019) Long-term Risk of Neuropsychiatric Disease After Exposure to Infection in Utero. JAMA Psychiatry. 76(6): 594-602.
-
Faye-Petersen OM, Heller DS, Joshi VV (2006) Handbook of Placental Pathology, 2nd London: Taylor & Francis.
-
Rombaldi RL, Serafini EP, Mandelli J, Zimmermann E, Losquiavo KP (2008) Transplacental transmission of human Virol J 5: 106.
-
Smith EM, Parker MA, Rubenstein LM, Haugen TH, Hamsikova E, et al. (2010) Evidence for vertical transmission of HPV from mothers to infants. Infect Dis Obstet Gynecol 2010: 326369.
-
Heller HT, Mullen KM, Gordon RW, Reiss RE, Benson CB (2014) Outcomes of pregnancies with a low-lying placenta diagnosed on second-trimester sonography. J Ultrasound Med 33(4): 691-696.
-
Wen SW, Demissie K, Liu S, Marcoux S, Kramer MS (2000) Placenta praevia and male sex at birth: results from a population-based Paediatr Perinat Epidemiol 14(4): 300-304.
-
Köstü B, Ercan Ö, Özer A, Bakacak M, Avcı F (2015) Male fetus domination in total placenta previa cases. Perinatal J 23: 84-88.
-
May T, Williams K (2018) Brief Report: Gender and Age of Diagnosis Time Trends in Children with AutismUsing Australian Medicare Data. J Autism Dev Disord 48(12): 4056-4062.
-
Human papillomavirus (HPV) and cervical cancer (2020) World Health Organization (WHO). Available online at: https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer
-
Chaturvedi AK, Engels EA, Pfeiffer RM, Hernandez BY, Xiao W, et (2010) Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 29: 4294-4301.
-
Autism Spectrum Disorder (ASD) Prevalence Studies and Autism spectrum disorder (ASD).
-
You H, Liu Y, Agrawal N, Prasad CK, Edwards JL, et (2008) Multiple human papillomavirus types replicate in 3A trophoblasts. Placenta 29: 30-38.
-
Weyn C, Thomas D, Jani J, Guizani M, Donner C, et (2011) Evidence of human papillomavirus in the placenta. J Infect Dis203(3): 341-343.
-
Walker CK, Anderson KW, Milano KM, Ye S, Tancredi DJ, et (2013) Trophoblast inclusions are significantly increased in the placentas of children in families at risk for autism. Biol Psychiatry 74(3): 204-211.
-
Children's National Health System. Placental function linked to brain injuries associated with autism. Science Daily. Accessed on January 15, 2020. Available online at: https://www.sciencedaily.com/releases/2019/04/190427104806.htm
-
Zuo Z, Goel S, Carter JE (2011) Association of cervical cytology and HPV DNA status during pregnancy with placental abnormalities and preterm Am J Clin Pathol 136: 260-265.
-
Fezer GF, Matos MB, Nau AL, Zeigelboim BS, Marques JM, et al. (2017) Perinatal features of children with autism spectrum disorder. Rev Paul Pediatr 35(2): 130-135.
-
Bruni L, Diaz M, Barrionuevo-Rosas L, Herrero R, Bray F, et al. (2016) Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 4(7): e453-e463.
-
Khan AT, Stewart KS (1987) Ultrasound placental localization in early pregnancy. Scott Med J 32(1): 19-21.
-
Rizos N, Doran TA, Miskin M, Benzie RJ, Ford JA (1979) Natural history of placenta previa ascertained by diagnostic ultrasound. Am J Obstet Gynecol 133(3): 287-291.
-
Cresswell JA, Ronsmans C, Calvert C, Filippi V (2013) Prevalence of placenta praevia by world region: a systematic review and meta-analysis. Trop Med Int Health 18(6): 712-724.
-
Kim LH, Caughey AB, Laguardia JC, Escobar GJ (2012) Racial and ethnic differences in the prevalence of placenta previa. J Perinatol 32(4): 260-264.
-
Füle T, Máthé M, Suba Z, Csapó Z, Szarvas T, et al. (2006) The presence of human papillomavirus 16 in neural structures and vascular endothelial cells. Virology 348(2): 289-296.
-
Chen J, Tsai V, Parker WE, Aronica E, Baybis M, et al. (2012) Detection of human papillomavirus in human focal cortical dysplasia type Ann Neurol 72: 881-892.
-
Millichap JG (2013) Focal Cortical Dysplasia Type IIB and Human Papillomavirus. Pediatr Neurol Briefs 27(3): 24-24.
-
Casanova MF, El-Baz AS, Kamat SS, Dombroski BA, Khalifa F, et al. (2013) Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathol Commun 1: 67.
-
de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H (2004) Classification of papillomaviruses. Virology 324(1): 17-27.
-
Arbeit JM, Münger K, Howley PM, Hanahan D (1993) Neuroepithelial carcinomas in mice transgenic with human papillomavirus type 16 E6/E7 ORFs. Am J Pathol 142(4): 1187-1197.
-
Praetorius J, Nejsum LN, Nielsen S (2004) A SCL4A10 gene product maps selectively to the basolateral plasma membrane of choroid plexus epithelial Am J Physiol Cell Physiol 286(3): C601-C610.
-
Sinning A, Liebmann L, Hübner CA (2015) Disruption of Slc4a10 augments neuronal excitability and modulates synaptic short-term Front Cell Neurosci 9: 223.
-
Gurnett CA, Veile R, Zempel J, Blackburn L, Lovett M, et al. (2008) Disruption of sodium bicarbonate transporter SLC4A10 in a patient with complex partial epilepsy and mental Arch Neurol 65(4): 550-552.
-
Shen MD, Kim SH, McKinstry RC, Gu H, Hazlett HC, et al. (2017) Increased extra-axial cerebrospinal fluid in high-risk infants who later develop autism. Biol Psychiatry 82(3): 186-193.
-
Schmitz M, Driesch C, Jansen L, Runnebaum IB, Dürst M (2012) Non-random integration of the HPV genome in cervical PLoS One 7(6): e39632.
-
Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, et (2007) Strong association of de novo copy number mutations with autism. Science 316(5823): 445-449.
-
Prasad GL, Mahapatra AK (2015) Case series of choroid plexus papilloma in children at uncommon locations and review of the Surg Neurol Int 6: 151.
-
Ogiwara H, Dipatrim AJ Jr, Aldenm TD, Bowmanm RM, Tomita T (2012) Choroid plexus tumors in pediatric Br J Neurosurg 26(1): 32-37.
-
Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA (2005) Neuroglial activation and neuroinflammation in the brain of patients with Ann Neurol 57(1): 67-81.
-
Shastri A, Bonifati DM, Kishore U (2013) Innate immunity and Mediators Inflamm 2013: 342931.
-
de Jonge WJ (2013) The Gut's Little Brain in Control of Intestinal ISRN Gastroenterol 2013: 630159.
-
Horvath K, Perman JA (2002) Autistic disorder and gastrointestinal Curr Opin Pediatr 14(5): 583-587.
-
Godar DE, Merrill SJ (2017) Untangling the most probable role for vitamin D3 in autism. Dermatoendocrinol 9(1): e1387702.
-
Godar DE, Gurov R, Merrill SJ (2017) All Sites but Skin Cancer Incidences Analyzed Worldwide by Sex, Age, and Skin Type over Time (1955-2007), Advancing Age, and UVB Dose Reveals Important Carcinogenic Drivers. J Epi Res 3(2): 65-80.
-
Crawley JN, Heyer WD, LaSalle JM (2016) Autism and cancer share risk genes, pathways, and drug targets. Trends Genet 32(3): 139-146.
-
Lee DY (2015) Roles of mTOR signaling in brain Exp Neurobiol 24(3): 177-185.
-
Tilot AK, Frazier TW II, Eng C (2015) Balancing proliferation and connectivity in PTEN-associated autism spectrum Neurotherapeutics 12(3): 609-619.
-
Spangle JM, Munger K (2010) The human papillomavirus type 16 E6 oncoprotein activates mTORC1 signaling and increases protein J Virol 84: 9398-9407.
-
Crino PB (2016) The mTOR signaling cascade: Paving new roads to cure neurological disease. Nat Rev Neurol 12(7): 379-392.
-
Sato A (2016) mTOR, a potential target to treat autism spectrum CNS Neurol Disord Drug Targets 15(5): 523-543.
-
Coppock JD, Wieking BG, Molinolo AA, Gutkind JS, Miskimins KW, et al. (2013) Improved clearance during treatment of HPV-positive head and neck cancer through mTOR Neoplasia 15(6): 620-630.
-
Yi JJ, Berrios J, Newbern JM, Snider WD, Philpot BD, et (2015) An autism-linked mutation disables phosphorylation control of UBE3A. Cell 162(4): 795-807.
-
Brimer N, Lyons C, Pol SBV (2007) Association of E6AP (UBE3A) with human papillomavirus type 11 E6 Virology 358(2): 303-310.
-
Bourgeron T (2015) From the genetic architecture to synaptic plasticity in autism spectrum Nat Rev Neurosci 16(9): 551-563.
-
Crespi B (2011) Autism and cancer Autism Res 4(4): 302-310.
-
Chiang HL, Liu CJ, Hu YW, Chen SC, Hu LY, et (2015) Risk of cancer in children, adolescents, and young adults with autistic disorder. J Pediatr 166(2): 418-423.
-
Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, et al. (2015) Preeclampsia, placental insufficiency and autism spectrum disorder or developmental JAMA Pediatr 169(2): 154-162.
-
Mc Donnold M, Dunn H, Hester A, Pacheco LD, Hankins GD, et (2014) High risk human papillomavirus at entry to prenatal care and risk of preeclampsia. Am J Obstet Gynecol 210(2): 1-5.
-
Fahmy FS, Sabri NA, El Hamamsy MH, El Sawi M, Zaki OK (2016) Vitamin D intake and sun exposure in autistic children. Int J Pharm Sci Res 7(3): 1043-1049.
-
Reddi AL, Sankaranarayanan K, Arulraj HS, Devaraj N, Devaraj H (2000) Serum alpha-N- acetylgalactosaminidase is associated with diagnosis/prognosis of patients with squamous cell carcinoma of the uterine Cancer Lett 158(1): 61-64.
-
von Essen MR, Kongsbak M, Schjerling P, Olgaard K, Odum N, et al. (2010) Vitamin D controls T cell antigen receptor signaling and activation of human T Nat Immunol 11: 344-349.
-
Wong CCY, Meaburn EL, Ronald A, Price TS, Jeffries AR, et (2014) Methylomic analysis of identical twins discordant for autism spectrum disorder and related behavioral traits. Mol Psychiatry 19(4): 495-503.
-
Siegel EM, Riggs BM, Delmas AL, Koch A, Hakam A, et al. (2015) Quantitative DNA methylation analysis of candidate genes in cervical PLoS One 10(3): e0122495.
-
Anayannis NVJ, Schlecht NF, Belbin TJ (2015) Epigenetic mechanisms of human papillomavirus-associated head and neck Arch Pathol Lab Med 139(11): 1373-1378.
-
Wentzensen N, Vinokurova S, von Knebel Doeberitz M (2004) Systematic review of genomic integration sites of human papillomavirus genomes in epithelial dysplasia and invasive cancer of the female lower genital Cancer Res 64(11): 3878-3884.
-
Akagi K, Li J, Broutian TR, Padilla-Nash H, Xiao W, et (2014) Genome-wide analysis of HPV integration in human cancers reveals recurrent, focal genomic instability. Genome Res 24: 185-199.
-
Smith CL, Bolton A, Nguyen G (2016) Genomic and epigenomic instability, fragile sites, schizophrenia and Curr Genomics 11(6): 447-469.
-
Cooper DN, Mort M, Stenson PD, Ball EV, Chuzhanova NA (2010) Methylation- mediated deamination of 5-methylcytosine appears to give rise to mutations causing human inherited disease in CpNpG trinucleotides, as well as in CpG Hum Genomics 4(6): 406-410.
-
Miranda PM, Silva NN, Pitol BC, Silva ID, Lima-Filho JL, et (2013) Persistence or clearance of human papillomavirus infections in women in Ouro Preto, Brazil. Biomed Res Int 2013: 578276.
-
Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, et (2012) Infant siblings and the investigation of autism risk factors. J Neurodev Disord 4(1): 7.
-
Zerbo O, Yoshida C, Gunderson EP, Dorward K, Croen LA (2015) Interpregnancy Interval and Risk of AutismSpectrum Disorders. Pediatrics 136(4): 651-657.
-
Maglennon GA, McIntosh PB, Doorbar J (2014) Immunosuppression facilitates the reactivation of latent papillomavirus infections. J Virol 88(1): 710-716.
-
Shim J, Pérez A, Symanski E, Nyitray AG (2016) Association Between Serum 25-Hydroxyvitamin D Level and Human Papillomavirus Cervicovaginal Infection in Women in the United States. J Infect Dis213(12): 1886-1892.
-
Lee BK, Eyles DW, Magnusson C, Newschaffer CJ, McGrath JJ, et al. (2019) Developmental vitamin D and autism spectrum disorders: Findings from the Stockholm Youth Cohort. Mol Psychiatry. Available online at: https://www.nature.com/articles/s41380-019-0578-y
-
Scheller NM, Pasternark B, Molgaard-Nielsen D, Svanström H, Hviid A (2017) Quadrivalent HPV Vaccination and the Risk of Adverse Pregnancy Outcomes. N Engl J Med 376: 1223-1233.
-
Holick MF (2015) Vitamin Dand brain health: The need for vitamin D supplementation and sensible sun exposure. J Intern Med 277(1): 90-93.
-
An HJ, Cho NH, Lee SY, Kim IH, Lee C, et (2003) Correlation of cervical carcinoma and precancerous lesions with human papillomavirus (HPV) genotypes detected with the HPV DNA chip microarray method. Cancer 97(7): 1672-1680.
-
Abreu AL, Souza RP, Gimenes F, Consolaro ME (2012) A review of methods for detect human papillomavirus Virol J 9: 262-270.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)








