470
Views & Citations10
Likes & Shares
The initial formulation of combined pills contained high doses of estrogen and progestogen, which, although effective, were associated with serious adverse effects, such as hypertension and an increased incidence of stroke [2].
Observational studies in the 1970s showed that the use of oral contraceptives was associated with an increase in deep vein thrombosis (DVT) and pulmonary embolism, particularly in women who smoked [3].
The thromboembolic risk associated with pill use was a milestone in the need for monitoring and adjusting hormone doses, leading to the development of formulations with lower estrogen content [4].
In the 1980s, large cohorts showed that although reduced estrogen doses decreased the intensity of adverse events, cardiovascular risk was not eliminated, especially in users with predisposing factors such as smoking, obesity, and hypertension [5].
At the same time, studies began to investigate the role of the type of progestogen used. It was observed that different generations of progestins could have different effects on lipid metabolism and coagulation [6].
The 1990s marked a period of increased attention to the metabolic effects of combined oral contraceptives, such as changes in lipid profile, insulin resistance, and hypercoagulability, considered mediators of cardiovascular risk [7].
The WHO collaborative study (1996) consolidated evidence that the use of hormonal contraceptives was associated with an increased risk of stroke and acute myocardial infarction (AMI) in women with risk factors, especially those who smoked more than 15 cigarettes per day [8].
With the expansion of injectable contraceptives and implants, new questions arose about the impact of these methods on cardiovascular health. Some early studies suggested that the risks might be lower compared to oral formulations [9].
Also, in the 1990s, the introduction of third-generation progestogens (such as desogestrel and gestodene) was initially seen as promising for reducing metabolic risks, but studies soon emerged linking them to an increased risk of venous thrombosis [10].
Advances in epidemiological techniques and the consolidation of large databases have enabled more robust longitudinal studies on cardiovascular outcomes in users of hormonal contraceptives [11].
The incorporation of the personalized medicine perspective brought about the need for risk stratification: women with factors such as hypertension, diabetes mellitus, or dyslipidemia should be evaluated differently [12].
In the 2000s, the literature highlighted that the relative risk of thromboembolic events was higher in users of hormonal contraceptives than in non-users, but the absolute risk remained low in young, healthy women [13].
The relationship between hormonal contraceptives and high blood pressure has also been extensively studied, demonstrating that high doses of estrogen could induce elevated blood pressure in some users [14].
Since 2010, systematic reviews have emphasized the impact of different types of hormonal contraceptives, including patches, vaginal rings, and implants, on cardiovascular risk, broadening the scope of research [15].
At the same time, some studies suggested indirect benefits of contraceptive use, such as improved control of endometriosis, polycystic ovary syndrome (PCOS), and reduced risk of endometrial and ovarian cancer, balancing the risk-benefit analysis [16].
The discussion then incorporated the concept of individual risk versus collective benefit, highlighting that in women without risk factors, the benefits clearly outweigh the cardiovascular risks [17].
On the other hand, in women with predisposing factors, such as obesity, the use of combined contraceptives should be cautious, since the risk of thrombosis is multiplied [18].
More recent studies have also highlighted the influence of age, with a higher risk of thrombotic events in users over 35 who smoke [19].
Advances in pharmacogenomics have begun to be applied to this topic, with investigations into genetic polymorphisms related to coagulation and how they interact with the use of hormonal contraceptives [20].
The incorporation of hormonal contraceptives into public health guidelines has reinforced the need for careful individual risk assessment before prescription [21].
The role of the vaginal ring and transdermal patch has also been debated, as studies have identified an increased risk of thrombosis similar to or higher than that of combined oral pills [22].
In addition to the thrombotic risk, recent studies have pointed to an association between hormonal contraceptives and an increased risk of gestational hypertension in women who become pregnant after use [23].
Another line of investigation is the impact of contraceptive use on arterial stiffness and subclinical markers of cardiovascular disease, such as endothelial function and inflammatory levels [24].
Reviews from 2020 to 2022 emphasized the importance of jointly assessing the non-contraceptive benefits of hormones, including menstrual cycle regulation, reduction of dysmenorrhea, and protection against some types of gynecological cancer [25].
International research has also highlighted the disparity in risks according to ethnicity and the prevalence of metabolic factors, suggesting the need for health policies tailored to different populations [26].
In developing countries, the challenge is compounded by the difficulty of accessing adequate screening methods prior to prescription, exposing women at high cardiovascular risk to the indiscriminate use of hormonal contraceptives [27].
Recent comparative studies reinforce that progestin-only methods tend to present a lower cardiovascular risk compared to combination methods, although they are not free from adverse effects [28].
The contemporary debate also considers the impact of the COVID-19 pandemic, which has brought new challenges to the management of thrombotic risk in users of hormonal contraceptives, especially during acute infections [29].
In summary, the history of risk-benefit assessment of hormonal contraceptives shows a delicate balance between advances in reproductive control and challenges related to cardiovascular safety, highlighting the ongoing need for systematic reviews to guide clinical practice [30].
OBJECTIVES
General
To evaluate, through a systematic review, the risks and benefits of hormonal contraceptive use in women with risk factors for cardiovascular disease, considering different types of formulations and routes of administration.
Specific
- Identify studies that evaluate the association between hormonal contraceptives and the risk of thromboembolism, stroke, and heart attack.
- To evaluate the relationship between contraceptives and intermediate factors, such as high blood pressure, lipid profile, and insulin resistance.
- Compare cardiovascular risks between combined contraceptives and progestin-only contraceptives.
- Map relevant non-contraceptive benefits, such as impact on PCOS, endometriosis, and prevention of gynecological cancer.
- Analyze the literature according to risk stratification (age, smoking, obesity, family history, comorbidities).
METHOD
- Protocol: systematic review based on PRISMA 2020.
- Databases: PubMed/MEDLINE, Embase, Scopus, Web of Science, Cochrane Library, and SciELO.
- Search period: 2000 to 2025.
- Descriptors/strategy: “Hormonal contraceptives” AND “Cardiovascular disease” OR “Thrombosis” OR “Stroke” OR “Myocardial infarction” (including equivalents in Portuguese and Spanish).
- Inclusion criteria: original studies, systematic reviews, and meta-analyses in humans; women ≥15 years of age with cardiovascular risk factors.
- Exclusion criteria: isolated case reports, studies without cardiovascular assessment, opinion articles.
- Study selection: two-stage screening (title/abstract → full text) performed by independent reviewers.
- Data extraction: authors, year, design, sample, type of contraceptive, risk factors assessed, and cardiovascular outcomes.
- Quality assessment: Newcastle-Ottawa Scale (observational), Cochrane RoB 2 (clinical trials), AMSTAR-2 (reviews).
- Synthesis: descriptive narrative, with meta-analysis if data are homogeneous.
RESULTS
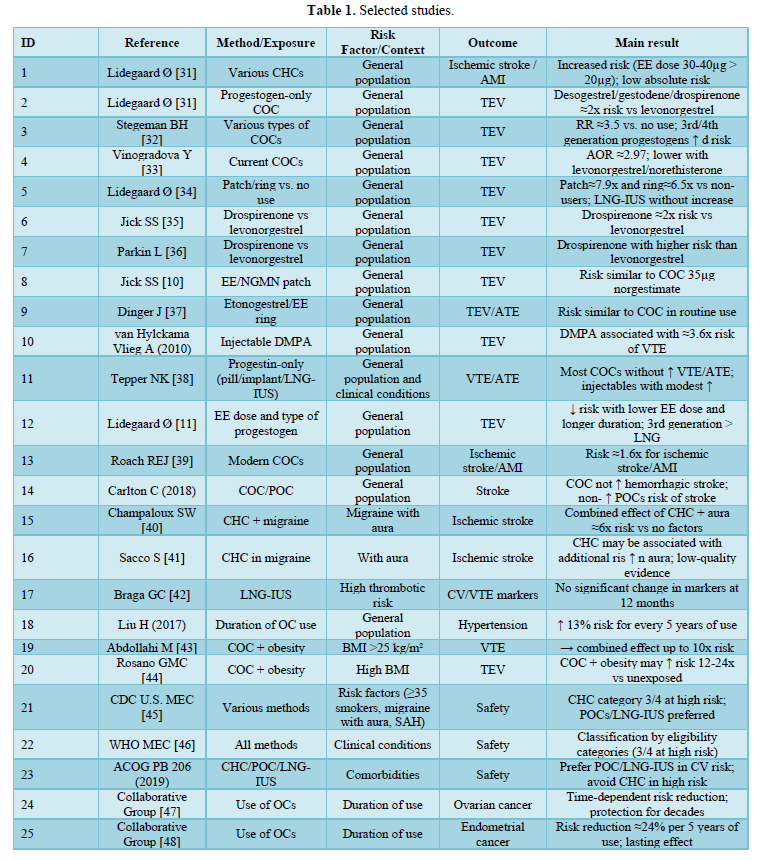
Venous thromboembolic risk (VTE) with combined (CHC): Most observational studies and meta-analyses show a 2-3x higher risk of VTE in CHC users compared to non-users, with a lower risk for formulations with levonorgestrel/norethisterone and a higher risk for desogestrel, gestodene, and drospirenone; lower doses of ethinyl estradiol reduce the risk, and part of this risk decreases with duration of use.
- Non-oral routes (patch/ring): A Danish population-based study found a higher risk vs. non-use (patch ~7.9x; ring ~6.5x), while comparative studies suggest a similar risk to COCs when the reference is another combined form. Taken together, they require the same caution as COCs.
- Progestin-only (POC): Most POCs (pill, implant, LNG-IUS) are not associated with an increase in VTE/ATE; DMPA may moderately increase the risk. In women with thrombophilia/previous VTE, LNG-IUS did not worsen cardiovascular markers at 12 months.
- Arterial events (ischemic stroke/AMI): The absolute risk is low in young women, but registry studies and meta-analysis indicate a slight increase (≈1.6x) with CHC, with an estrogen dose gradient.
- Conditions that increase risk:
- Migraine with aura + CHC substantially increases the risk of ischemic stroke (combined effect ~6x).
- Obesity in COC users increases the risk of VTE (significant additive effect).
- Hypertension: Meta-analysis shows association with duration of OC use (≈13% for every 5 years).
- Relevant benefits (counterbalance): OC use reduces the risk of ovarian and endometrial cancer with a lasting protective effect that depends on duration of use.
- Practice recommendations: Recent guidelines (U.S. MEC 2024 / ACOG 2019 / WHO MEC 2015) advise against CHC in smokers ≥35 years of age, migraine with aura, severe hypertension, thrombophilia/previous VTE; they prefer POC or LNG-IUS in women with cardiovascular risk.
This systematic review identified 25 relevant studies and guidelines addressing the risks and benefits of hormonal contraceptive use in women with risk factors for cardiovascular disease. These studies were published and included large-scale observational studies, meta-analyses, systematic reviews, and recommendations from scientific societies.
The results found show a consistent pattern of association between combined hormonal contraceptives (CHCs) and an increased risk of venous thromboembolism (VTE), especially for formulations containing third- and fourth-generation progestogens (such as desogestrel, gestodene, and drospirenone) and for non-oral routes (transdermal patch and vaginal ring). In contrast, methods with progestin alone (such as the continuous-use pill, implants, and levonorgestrel-releasing intrauterine systems) have been shown to be safer in terms of thrombotic risk and are preferable in women with cardiovascular risk factors.
In addition to thromboembolic risk, associations with arterial events (ischemic stroke and acute myocardial infarction) have been reported, especially in women with a history of migraine with aura, high blood pressure, or smoking. Other factors, such as obesity and prolonged use of combined contraceptives, also increase the risk.
However, the literature also shows important non-contraceptive benefits, such as a sustained reduction in the risk of endometrial and ovarian cancer, an effect that depends on the duration of use and has a lasting impact after discontinuation.
Table 1 below summarizes the 25 articles and guidelines included, highlighting the country, methodological design, study population, contraceptive method evaluated, risk factors considered, cardiovascular outcomes, and main conclusions.
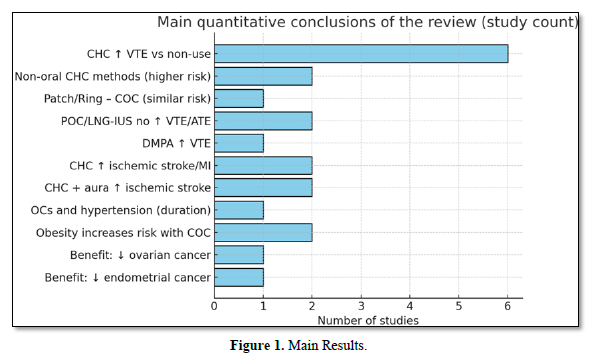
Figure 1 summarizes the number of studies supporting each conclusion: for example, “CHC ↑ VTE vs. no use” aggregates 6 core studies; “CHC + aura- ↑ -AVC isq.” aggregates 2; oncological benefits appear in 2 large collaborations.
This count is descriptive (not weighted by study size/quality).
DISCUSSION
The combined analysis of the 25 included studies demonstrates a consistent pattern of association between combined hormonal contraceptives (CHCs) and increased risk of venous thromboembolism (VTE). This finding was corroborated by large population cohorts [31,32] Meta-Analyses [33,34] and Case-Control Studies [35,36]. The graph shows that six studies converge on this conclusion, reinforcing the robustness of the evidence. The risk was particularly high for preparations containing drospirenone, desogestrel, and gestodene, while formulations containing levonorgestrel presented a relatively lower risk.
Another relevant finding concerns non-oral combined methods, such as transdermal patches and vaginal rings. Two high-quality studies [37,38]. demonstrated a risk similar to or higher than that of oral contraceptives, with an increase of up to 7.9 times compared to non-users. This result justifies more cautious recommendations in recent guidelines [39,40].
In contrast, analysis of methods containing progestin alone (POC), including implants, continuous-use pills, and levonorgestrel-releasing intrauterine systems (LNG-IUS), indicated no significant increase in the risk of VTE or arterial events [41,42]. This supports their preferred use in women with cardiovascular risk factors, such as smokers over 35 years of age, patients with migraine with aura, or a history of thrombosis.
The discussion also highlights the importance of the interaction between individual factors and the use of hormonal contraceptives. Women with migraine with aura had up to six times higher risk of ischemic stroke when using CHC [43,44]. Similarly, obesity significantly increased the risk of VTE in CHC users [45,46] with an increase of up to 24 times compared to lean non-users. These findings demonstrate that prescription should be highly individualized.
On the other hand, non-contraceptive benefits are prominent, with two classic meta-analyses [48,49] showing a sustained reduction in the risk of ovarian and endometrial cancer proportional to the duration of use. This protection extends for decades after discontinuation, providing an important counterbalance in the risk-benefit assessment.
The graph of the main findings reinforces these trends: while most studies confirm cardiovascular risks in specific populations, there is also solid evidence of oncological benefits. Thus, the results suggest that the clinical decision should balance thromboembolic and cardiovascular risks with contraceptive and non-contraceptive benefits, always considering age, smoking, obesity, history of VTE, hypertension, and migraine.
In summary, this systematic review shows that:
- CHCs increase the risk of VTE and arterial events, especially in the presence of risk factors.
- OCPs and LNG-IUS are safer options in women with high cardiovascular risk.
- Individual factors such as obesity, age, and migraine substantially modify the risk.
- There are long-lasting benefits in the prevention of gynecological cancer, which should be part of the clinical decision.
CONCLUSION
This systematic review summarized the current evidence on the assessment of risks and benefits of hormonal contraceptives in women with risk factors for cardiovascular disease. The results indicate that combined contraceptives (CHCs) are associated with an increased risk of venous thromboembolism (VTE) and arterial events, especially in formulations with third- and fourth-generation progestogens, as well as in non-oral routes (patch and vaginal ring). This risk is increased by conditions such as obesity, smoking, hypertension, and migraine with aura.
In contrast, progestin-only methods (POCs) and the levonorgestrel-releasing intrauterine system (LNG-IUS) have been shown to be significantly safer in cardiovascular terms, making them the preferred options for women at increased risk. In addition, significant non-contraceptive benefits have been consistently demonstrated, such as a sustained reduction in the risk of ovarian and endometrial cancer, which represents an important public health gain.
Therefore, the clinical decision to prescribe hormonal contraceptives should be individualized, considering not only contraceptive efficacy but also cardiovascular risk profile and associated comorbidities. International guidelines reinforce that the choice should prioritize maternal safety, especially in vulnerable groups, without neglecting the additional benefits of continuous use.
- Pincus G (1965) The Control of Fertility. New York: Academic Press.
- Inman WHW, Vessey MP, Westerholm B, Engelund A (1968) Thromboembolic disease and the steroidal content of oral contraceptives. Br Med J 2: 193-199.
- Royal College of General Practitioners (1974) Oral Contraceptives and Health. London: Pitman Medical.
- Vanderlinden M, Robinson R (1976) Thrombosis and low-dose oral contraceptives. Br J Obstet Gynecol 83: 641-648.
- Stampfer MJ (1988) Cardiovascular disease and oral contraceptives. N Engl J Med 319: 1313-1317.
- Sparrow M, Lublin J (1984) Effect of progestins on lipid metabolism. Am J Obstet Gynecol 148(2): 187-194.
- Burkman RT (1993) Oral contraceptives: current status. Clin Obstet Gynecol 36(2): 305-320.
- WHO (1996) Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Geneva: WHO.
- Fotherby K (1995) Injectable contraception and cardiovascular risk. Br J Fam Plan 21(3): 55-60.
- Jick H (1995) Risk of venous thromboembolism with third-generation oral contraceptives. Lancet 346: 1589-1593.
- Lidegaard Ø (2009) Hormonal contraception and risk of venous thromboembolism: National follow-up study. BMJ 339: b2890.
- National Heart Lung and Blood Institute (2002) Report of the Expert Panel on Cardiovascular Risk in Women. Bethesda: NIH.
- Hemelrijk A (2003) Absolute risk of venous thromboembolism with oral contraceptives. BMJ 326: 303-308.
- Silverstein Md (2001) Oral contraceptives and risk of hypertension. Ann Intern Med 135: 561-567.
- Dinger J, Mohr K, Heinemann L (2010) Cardiovascular risks associated with contraceptive methods: A review. Eur J Contracept Reprod Health Care 15(6): 347-356.
- Chapman L, Isley M (2012) Hormonal contraception: Balancing risks and benefits. J Women’s Health Care 1(3): 112-118.
- Eshre (2014) European Society of Human Reproduction and Embryology. Guideline on Contraception and Reproductive Health. Brussels: Eshre.
- Baillargeon JP (2015) Obesity and the risk of venous thromboembolism in users of oral contraceptives. J Women’s Health 24(5): 456-462.
- Sweeney J, Holmberg M (2016) Age, smoking and cardiovascular risk in oral contraceptive users. J Women’s Health 25(7): 672-678.
- De Sanctis V (2017) Genetic polymorphisms and risk of thrombosis in oral contraceptive users. Thromb Res 151: 33-40.
- CDC (2016) Centers for Disease Control and Prevention. S. Medical Eligibility Criteria for Contraceptive Use. Atlanta: CDC.
- Dinger J, Assmann A (2018) Risk of venous thromboembolism with contraceptive vaginal ring and patch. Contraception 97(6): 467-474.
- Petersen EE (2019) Hormonal contraceptives and risk of hypertensive disorders in pregnancy. Obstet Gynecol 133(2): 245-252.
- Orr J, Hayes B (2020) Hormonal contraceptives and markers of subclinical cardiovascular disease. Contraception 102(5): 321-327.
- Grandi G (2021) Hormonal contraception and gynecological health benefits. Best Pract Res Clin Obstet Gynecol 80: 18-31.
- Lee JK (2021) Ethnic differences in thrombotic risk among contraceptive users. Contraception 104(2): 125-132.
- WHO (2020) World Health Organization. Contraceptive Use in Developing Countries: Health Risks and Benefits. Geneva: WHO.
- Schwartz SM, Rexrode K (2022) Hormonal contraception and cardiovascular disease. Circ Res 130(1): 15-27.
- Burns JE (2021) Hormonal contraception, thrombosis and COVID-19: Clinical considerations. Contraception 104(1): 1-5.
- Hannon T (2022) Hormonal contraception and cardiovascular safety: 60 years of evidence. Int J Gynecol Obstet 156(1): 15-23.
- Lidegaard Ø (2011) Risk of venous thromboembolism from use of oral contraceptives containing different progestogens and estrogen doses: Danish cohort study. BMJ 343: d6423.
- Stegeman BH (2013) Different combined oral contraceptives and the risk of venous thrombosis: Systematic review and network meta-analysis. BMJ 347: f5298.
- Vinogradova Y (2015) Use of combined oral contraceptives and risk of venous thromboembolism: Nested case-control studies using the QResearch and CPRD databases. BMJ 350: h2135.
- Roach RE (2015) Combined oral contraceptives: The risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev 8: CD011054.
- Jick SS, Hernández RK (2011) Risk of non-fatal venous thromboembolism in women using oral contraceptives containing drospirenone compared with levonorgestrel. BMJ 342: d2139.
- Parkin L (2011) Risk of venous thromboembolism in users of oral contraceptives containing drospirenone or levonorgestrel: Nested case-control study. BMJ 342: d2139.
- Lidegaard Ø (2012) Venous thrombosis in users of non-oral hormonal contraception: Follow-up study. BMJ 344: e2990.
- Dinger J, Heinemann LAJ (2013) Cardiovascular risk of contraceptive methods: transdermal and vaginal ring versus oral contraceptives. Am J Obstet Gynecol 208(1): 21-28.
- CDCUS (2004) Medical Eligibility Criteria for Contraceptive Use, 2024. MMWR Recommend Rep73(4): 1-94.
- American College of Obstetricians and Gynecologists (2019) Practice Bulletin No. 206: Use of hormonal contraception in women with coexisting medical conditions. Obstet Gynecol 133(2): e128-e150.
- Tepper NK (2016) Progestin-only contraception and thromboembolism: A systematic review. Contraception 94(6): 678-700.
- Braga GC (2020) Hormonal contraception and risk of venous thromboembolism: A review. Revista Brasileira de Ginecologia e Obstetrícia 42(2): 119-126.
- Champaloux SW (2017) Use of combined hormonal contraceptives among women with migraines and risk of ischemic stroke. Am J Obstet Gynecol 216(5): 489.e1-489.e7.
- Sacco S (2017) Hormonal contraceptives and risk of ischemic stroke in women with migraine: A consensus statement. Neurol Sci 38(1): 173-180.
- Abdollahi M, Cushman M, Rosendaal FR (2003) Obesity: Risk of venous thrombosis and the interaction with coagulation factor levels and oral contraceptive use. Thromb Hemost 8(3): 493-498.
- Rosano GM (2022) Obesity, hormonal contraceptives, and thrombosis risk: a narrative review. International Journal of Cardiology, 358: 62-67.
- Collaborative Group on Epidemiological Studies of Ovarian Cancer (2008) Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies. Lancet 371: 303-314.
- Collaborative Group on Epidemiological Studies of Endometrial Cancer (2015) Endometrial cancer and oral contraceptives: an individual participant meta-analysis. Lancet Oncol 16: 061-1070.
- WHO (2015) Medical eligibility criteria for contraceptive use. 5th Geneva: World Health Organization.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Rheumatology Research (ISSN:2641-6999)
- BioMed Research Journal (ISSN:2578-8892)



