1756
Views & Citations756
Likes & Shares
The findings indicate no significant association between progesterone blood levels at embryo transfer and pregnancy outcomes. Additionally, patients receiving supplemental SP had similar pregnancy rates to patients with P4>30nmol/L and patients who received SP due to a previous failed FET cycle. Additionally, though non-significant, there was an association between higher pregnancy rates and the use of Utrogestan pessaries compared to Cyclogest. These results signal that additional SP could be beneficial for patients with a previous failed FET cycle, however, further large-scale studies are warranted to investigate this.
Overall, this study contributes to the ongoing debate regarding luteal phase support strategies in FET cycles, highlighting the need for individualized hormone monitoring and supplementation strategies.
Abbreviations: ANOVA: Analysis of Variance; BMI: Body Mass Index; CI: Confidence Interval; HCP: Healthcare Professional; OR: Odds Ratio; SAP: Statistical Analysis Plan; SD: Standard Deviation
Given the significance of P4 in reproduction, there is growing interest in determining optimal systemic P4 levels during HRT-FET cycles to enhance the success of assisted reproductive techniques (ART) [2]. Recent studies suggest that serum P4 levels below 9.2 ng/ml (29.3nmol/L) or 10 ng/ml (31.8 nmol/L) on the day of embryo transfer negatively impact pregnancy rates [2]. In cases of insufficient progesterone, known as luteal phase defect, inadequate progesterone levels may prevent normal secretory endometrial development, thereby hindering embryo implantation and growth [1].
The number of FET procedures has been increasing worldwide over the last decade [3]. The adoption of "freeze-all" policies-implemented to mitigate the risk of ovarian hyperstimulation syndrome and the adverse effects of supraphysiologic estradiol (E2) levels and premature progesterone elevation-has contributed to the growing demand for FET cycles [3]. Despite this, there is limited evidence for the use of additional progesterone in patients with previous failed cycles. This study aims to assess the pregnancy outcomes of 2 different strategies for luteal phase support; adding additional subcutaneous progesterone (SP, Lubion, 25mg, qd) in patients below a P4 threshold of 30nmol/L on the day of embryo transfer (ET), and in patients with a previous failed FET cycle, regardless of progesterone level.
STUDY AIM AND OBJECTIVES
Research question
What is the association between progesterone blood levels and pregnancy outcome following embryo transfer?
Primary objective
To investigate whether progesterone blood levels at the time of embryo transfer are associated with pregnancy outcome following embryo transfer.
Secondary objectives
- To investigate whether use of SP for patients with P4<30nmol/L on the day of ET will result in similar pregnancy outcomes to patients with P4>30nmol/L.
- To investigate whether patients receiving SP due to P4 < 30nmol/L have a similar odds of positive pregnancy outcome compared to patients given Lubion due to previous failed FET cycle.
- To investigate the impact of progesterone levels, BMI, age and the type of vaginal pessary (Utrogestan and Cyclogest) on pregnancy outcomes.
METHODS AND MATERIALS
This observational study was conducted at the London Women's Clinic, Infertility Centre in Darlington, United Kingdom, from January 2023 to December 2023. Informed consent for non-contact research was obtained from all participants before inclusion. All patients underwent artificial endometrial preparation prior to FET, utilizing progesterone pessaries (Cyclogest, 400mg, BD or Utrogestan, 200mg, TDS) with or without progesterone injections. Serum progesterone levels were assessed on the day of embryo transfer, and additional P4 supplementation (Lubion, 25mg, OD) was administered when levels were found to be suboptimal (
Inclusion and Exclusion Criteria
Participants included women younger than 45 years with a BMI
Hormone Replacement Protocol
For artificial FET cycles, estradiol (8 mg daily) was initiated on day 2 of the menstrual cycle. An ultrasound examination was performed on day 10, and if the endometrial thickness was ≥7 mm, embryo transfer was scheduled for day 5 of progesterone administration (initiated on day 10 of the cycle). VP was either Cyclogest (400mg, BD) or Utrogestan (200mg, TDS). In patients with P4<30nmol/L on the day of embryo transfer, Lubion (25mg, OD) was added. In patients with a previous failed FET cycle or with a missing previous history, VP was administered alongside Lubion (25mg, once daily) from 5 days prior to embryo transfer. All patients received progesterone until the 12th week of gestation.
A blood sample was obtained on the day of embryo transfer to verify that progesterone levels met the target threshold (≥30 nmol/L). Embryos were generated through in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI), and graded using the Gardner criteria. Embryo transfer was performed under ultrasound guidance. A home pregnancy test was conducted on day 10 post-transfer.
Statistical Analysis
Data were cleaned and analyzed using R studio version 4.2.3. Descriptive statistics were computed and described before analyses for primary and secondary research objectives were conducted.
A multivariate logistic regression model was built with progesterone as the predictor variable and pregnancy outcome (positive or negative) as the outcome variable. Models included age, BMI, and a categorical concomitant treatment variable as covariates. Results are reported as odds ratio (OR) with 95% CI of positive pregnancy test (given a one-point increase in progesterone).
For analysis of secondary objective 1, patients recorded as having received SP prior to their progesterone level test were excluded. Differences in mean BMI, age, and progesterone results between patients received SP and patients not received SP was compared using T-tests. Differences in the proportion of positive pregnancy tests and other concomitant medications between those two groups was compared using Chi-squared tests. For analysis of secondary objective 2, All patients, including those received SP prior to testing, were included. Differences in mean BMI, age, and progesterone results between patients received SP prior to receipt of progesterone blood test results, patients received Lubion following receipt of progesterone blood test results, and patients not received Lubion were compared using one-way ANOVA. Differences in the proportion of positive pregnancy tests and other concomitant medications between these groups were compared using Chi-squared tests.
Another multivariate logistic regression model was built with Lubion prescription (pre- test, post-test, not received) as a predictor, and pregnancy outcome as outcome. BMI, age and concomitant prescriptions (other than Lubion) were included as covariates. Progesterone level test results were not included as a covariate as they are likely to be correlated with Lubion provision prior to testing. Results are reported as OR with 95% CI of a positive pregnancy test for each Lubion prescription condition.
RESULTS
Patient Characteristics
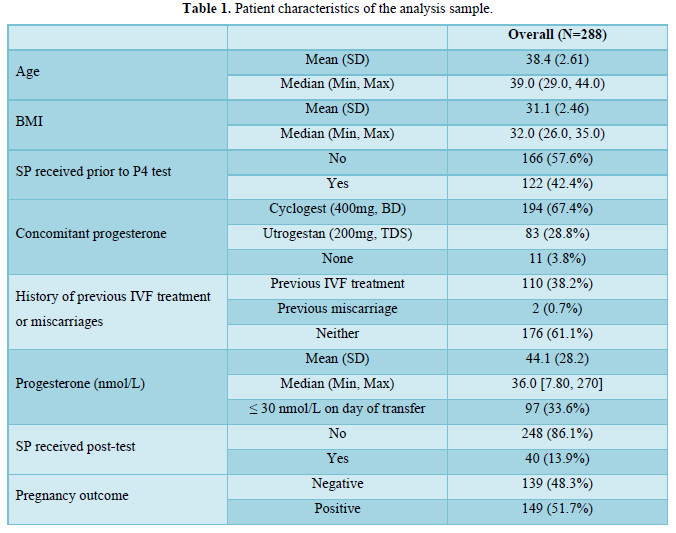
From a total sample of 290 patients, 288 were included in the analysis sample. Two patients were excluded due to: missing information on pregnancy outcome (n=1) and missing information on concomitant medications (n=1).
Patient characteristics of the analysis sample are described in Table 1.
Primary Objective
Results of the logistic regression analyses are presented in Table 2. Univariate logistic regression analyses suggested that progesterone blood levels at embryo transfer were not significantly associated with pregnancy outcome following embryo transfer. Controlling for age, BMI and concomitant vaginal progesterone (VP) medication did not significantly influence the results.

Secondary Objectives
Patients with Low Serum P Levels and iLPS vs. Patients with Normal Serum P Levels
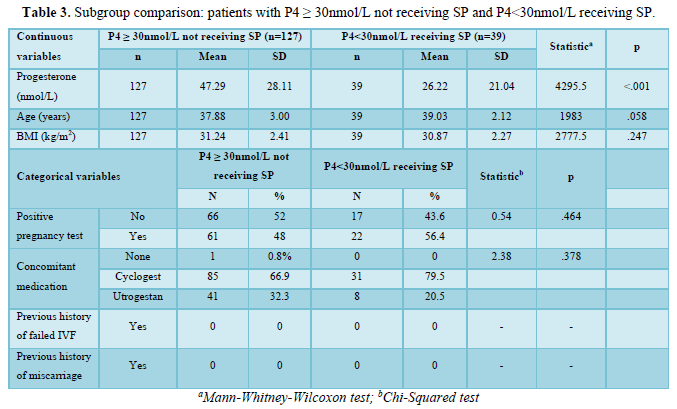
A total of 122 patients were excluded from this analysis due to prescription of Lubion prior to test, which may have impacted progesterone blood levels at transfer. A total of 166 participants were included.
Patient characteristics for this analysis are presented in Table 3. Progesterone blood levels at transfer were significantly lower in patients who were received subcutaneous progesterone following test results. There were no other significant differences between groups. All patients with a history of IVF or miscarriage had received Lubion prior to test, and were therefore not included in this analysis.
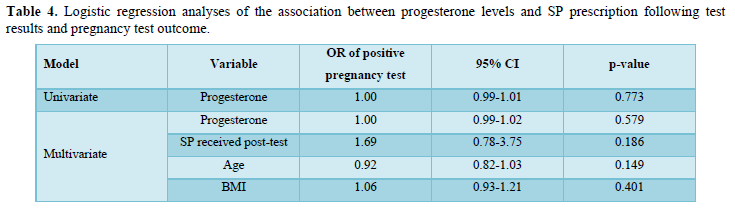
Logistic regression analyses results are presented in in Table 4. Univariate logistic regression analyses suggested that progesterone blood levels at transfer were not significantly associated with pregnancy outcome following embryo transfer in those not previously received Lubion. Lubion prescription did not significantly influence the non-significant association between progesterone and pregnancy outcome. Controlling for age and BMI also did not significantly influence the results.
Pregnancy outcomes in patients received SP for P4<30nmol/L or for previous failed cycle or miscarriage
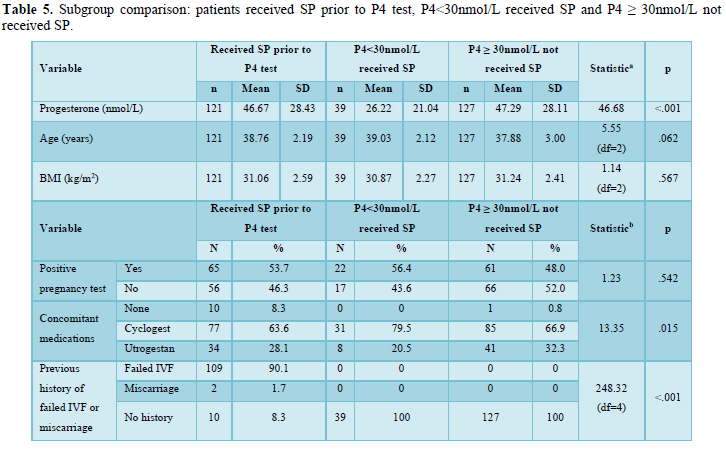
One individual was excluded from analyses of secondary objective 2 due to prescription of SP both before and after progesterone blood level results. A total of 287 participants were included.
Patient characteristics for this analysis are shown in Table 5. There were significant differences in progesterone blood levels between the three groups (p<.001). Other concomitant medications received and history of IVF treatment or miscarriage also differed between groups (p=.015 and p<.001, respectively). There were no other significant differences between groups.
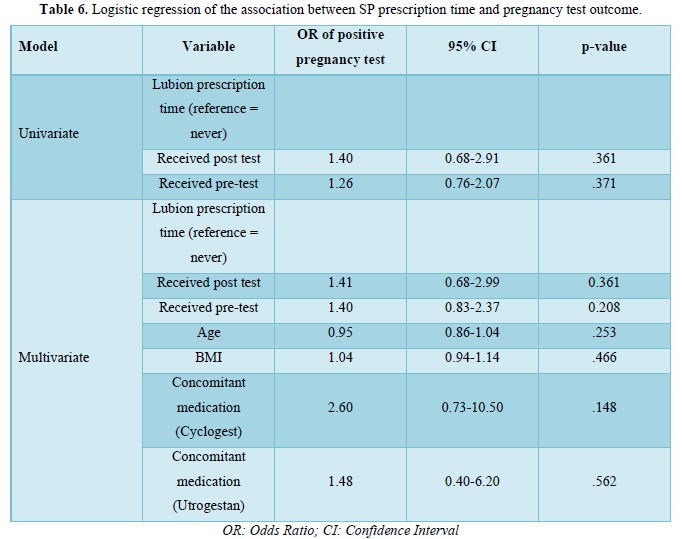
 Logistic regression analyses are presented in Table 6. Univariate logistic regression analyses suggested that SP prescription, either prior to, or after, progesterone blood level test results was not significantly associated with pregnancy test outcome. Controlling for age, BMI and other concomitant medications did not significantly influence the results.
Logistic regression analyses are presented in Table 6. Univariate logistic regression analyses suggested that SP prescription, either prior to, or after, progesterone blood level test results was not significantly associated with pregnancy test outcome. Controlling for age, BMI and other concomitant medications did not significantly influence the results.
DISCUSSION
This observational study provides insights into the role of progesterone levels and supplementation strategies in frozen embryo transfer (FET) cycles. The results demonstrated no significant difference in pregnancy rates between patient groups, and no association to progesterone level on the day of ET. This indicates that in patients with adequate P4 (≥30nmol/L), higher progesterone levels won’t result in an increased pregnancy rate. This result has been demonstrated in previous studies such as Ramos et al. in which patients who received SP alongside VP had no significant differences in clinical pregnancy rate varying across the quartiles of progesterone levels on the day of ET (2020). However, it should be noted that the patients in the top 50% of progesterone levels had significantly lower miscarriage rates. There could be a potential role of higher serum progesterone levels for miscarriage rate which was not examined in our study.
There were several other findings that offer valuable implications for clinical practice in assisted reproductive technologies (ART). First, the subgroup of patients with progesterone levels
Secondly, the comparison of pregnancy outcomes between patients receiving SP before versus after P4 measurement highlighted an important clinical consideration. Although not statistically significant, there was a trend towards higher success in patients treated pre-emptively due to prior failed FET cycles. This finding suggests that clinical history might serve as a surrogate marker for identifying patients at risk of inadequate luteal phase support and may guide empirical supplementation strategies [6]. Further research is warranted in this area to elucidate these findings.
Moreover, the association between the use of Cyclogest and higher pregnancy rates compared to the use of Utrogestan suggests that formulation and route of administration may play a role in treatment outcomes. Differences in pharmacokinetics between Cyclogest and Utrogestan could underlie this observation, though further research is warranted to confirm any differential effects [7].
Implications for Clinical Practice
These findings underscore the necessity for individualized luteal support strategies in FET cycles. Rather than adopting a one-size-fits-all threshold, a combination of serum hormone measurements and patient history may better identify those likely to benefit from additional progesterone support. Furthermore, rigid serum P4 thresholds (e.g., 30 nmol/L) may not be the best indicator for supplementation decisions, and further research to try and identify optimal and individualized thresholds would be beneficial.
Healthcare professionals might consider earlier or empirical intervention in select high-risk populations, such as those with previous implantation failure or miscarriage, even in the absence of real-time progesterone serum level data. Additionally, further exploration of the optimal route and formulation of progesterone could enhance treatment personalization.
Future Research Directions
Future studies should focus on prospective, randomized controlled trials stratifying patients based on both serum P4 levels and clinical history. Such trials would ideally include arms comparing different progesterone formulations, routes of administration, and initiation timing to better understand their influence on implantation and pregnancy outcomes. Further research to understand the optimal luteal support strategy for patients with previous failed FET cycles are needed.
In addition, mechanistic studies are warranted to elucidate why certain patients respond differently to similar progesterone levels and to explore biomarkers beyond serum progesterone that could guide therapy. Longitudinal studies could also assess the impact of individualized luteal support strategies on live birth rates and perinatal outcomes.
CONCLUSION
The study identified that pregnancy outcomes remained comparable between groups, suggesting that timely supplementation may offer a window of opportunity to optimize hormonal support and potentially salvage cycles with initially low progesterone levels or in patients with previously failed cycles.
Given the increasing use of FET cycles in assisted reproductive technology (ART), tailored progesterone supplementation strategies remain crucial. Future large-scale, randomized controlled trials are necessary to establish definitive progesterone thresholds and optimize clinical protocols for improving pregnancy success rates in FET cycles.
- Csapo AI, Pulkkinen MO, Wiest WG (1973) Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol 115(6): 759-765.
- Melo P, Chung Y, Pickering O, Price MJ, Fishel S, et al. (2021) Serum luteal phase progesterone in women undergoing frozen embryo transfer in assisted conception: A systematic review and meta-analysis. Fertil Steril 116(6): 1534-1556.
- Singh B, Reschke L, Segars J, Baker VL (2020) Frozen-thawed embryo transfer: The potential importance of the corpus luteum in preventing obstetrical complications. Fertil Steril 113(2): 252-257.
- Gaggiotti-Marre S, Martinez F, Coll L, García S, Álvarez M, et al. (2019) Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles. Reprod BioMed Online 38(5): 699-706.
- Labarta E, Mariani G, Holtmann N, Celada P, Remohí J, et al. (2021) Low serum progesterone on the day of embryo transfer is associated with lower ongoing pregnancy rates in artificial cycles: A systematic review and meta-analysis. Human Reprod 36(7): m1950-m1964.
- Alsbjerg, B, Polyzos NP, Elbaek HO, Laursen RJ, Povlsen BB, et al. (2018) Progesterone supplementation in artificial cycles is associated with higher live birth rates: A retrospective cohort study. Reprod BioMed Online 36(5): 635-642.
- van der Linden M, Buckingham K, Farquhar C, Kremer JAM, Metwally M (2015) Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015(7): CD009154.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Chemotherapy Research Journal (ISSN:2642-0236)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Pathology and Toxicology Research


