6450
Views & Citations5450
Likes & Shares
Result: There was generally poor knowledge about HCV among the participants. Only 31% of participants had good knowledge. Good knowledge was significantly associated with education and occupation of respondents (P=0.000). Being a ‘health worker’ (O.R = 0.3, CI= 0.15-0.81, P=0.010) and a ‘professional/civil servant’ (O.R= 0.4, CI = 0.22-0.83, p= 0.010) were significant predictors of good knowledge. The prevalence of HCV was 1.5%. Only 5.3 % of the study subject was screened in their index pregnancy.
Conclusion: There was poor knowledge of HCV infection, low level of access to HCV screening among participants and a prevalence of 1.5%. Considering the socioeconomic and health burden of Hepatitis C virus infection in Nigeria, there is the need for a policy change towards routine screening of pregnant women.
Keywords: Knowledge, Hepatitis C virus, Access to screening, Prevalence, Pregnant women, Antenatal care
Globally, the prevalence of HCV infection in pregnancy ranges from 1-8 % [6], while mother to child transmission of HCV ranges from 4-8 % [7]. Vertical transmission of Hepatitis C virus infection during pregnancy occurs mainly around the time of delivery [8] and is associated with increased risk of both maternal and fetal complications which may include vaginal bleeding, placental separation, preterm rupture of membrane and preterm delivery [9,10]. Hence, prevention of transmission of HCV in pregnancy is very vital [7].
Due to lack of awareness and knowledge of HCV infection on the part of patient and provider as well as inconsistent guidelines for screening, this disease has largely gone unnoticed [11]. As a result, 75% of individuals infected with HCV are not aware of their disease condition [12,13] and they are therefore at a high risk of developing complications of the infection without having the opportunity for proper treatment and management of the disease as well as transferring the disease to their unborn generations.
The first step towards identifying individuals with HCV infection is through screening. Health care providers need to have the ability to conduct and offer screening or at least refer individuals for screening in order to make more people know about their HCV status. Improving the access of pregnant women to HCV screening through promoting routine availability of HCV screening during antenatal care will lead to continuum of care, early diagnosis and start of treatment and finally, reduction of morbidities and mortalities due to HCV infection [14].
In Nigeria, routine diagnosis of HCV infection is limited to the people who present for blood donation with limited number of women presenting as donors in the country [15]. Again, in most health care centers, routine antenatal screening tests are limited to that of hepatitis B virus, HIV, syphilis and not HCV, yet antenatal care would have provided a ‘single window of opportunity to give a thorough counseling as well as proper screening for a wide range of infectious diseases including HCV. It has also been noted that factors responsible for increased prevalence of HCV infection include low knowledge on the part of the patients and the health care providers, poor health seeking behavior as well as inadequate screening [16]. The aim of this study is therefore to assess the level of knowledge about HCV, the prevalence and the access/utilization of HCV screening among pregnant women attending antenatal clinic in Lagos state, Nigeria.
MATERIALS AND METHODS
Study area/setting
Lagos State with a population estimate of 17,552942 people and population density of 5000 persons/kilometer is one the densely populated cities in the African continent [17]. The Island Maternity Hospital Lagos is one of the main maternity hospitals in Nigeria. The hospital contains 215 hospital beds and a special Care baby Unit. Lagos Island maternity hospital is a specialist hospital with over 7000 antenatal attendees yearly and takes care of obstetrics and gynecological cases in Lagos. It receives referrals from both private and government health care centers around the city [18].
Study population
Women receiving antenatal care at the Lagos Island Maternity Hospital were the study's subjects. The majority of these ladies were from various parts of the country but resides in Lagos for one economic benefit or the other making these women a suitable population for the study.
Study design
A cross-sectional survey was conducted. Between March and October 2015, consenting antenatal patients at the Lagos Island Maternity facility were selected, and their blood samples were collected for analysis. Information on participants' socio-demographic characteristics, knowledge about HCV infection as well as access to screening and prior utilization of HCV screening was gathered using a pretested semi-structured questionnaire.
Sample size calculation
This was determined using prior research that found the prevalence of HCV among pregnant women receiving antenatal care in Southwest Nigeria to be 3.9%, with a margin of error of plus or minus 5% [19]. After accounting for a 10% non-response rate, 400 pregnant women in total were enrolled.
Exclusion/ Inclusion criteria
All pregnant women older than or equal to 15 years, who were receiving antenatal care and who signed informed consent were recruited. Those that refused consent or were very ill were excluded from the study.
Ethical considerations
The ethical committee of Lagos State Government/ Lagos State University Teaching Hospital, Ikeja gave ethical approval for the study with ethical certificate number LREC/10/06/531. Written Informed consent was obtained from every study participant before recruitment. Anonymity, confidentiality and privacy were adhered to and participation was strictly voluntary.
Data collection
Quantitative methodology was used for data collection. Data was gathered using laboratory testing as well as a semi-structured interviewer administered questionnaires. The serologic data and questionnaires were linked using serial numbers and individual identity and codes. Questions testing patient knowledge on modes of transmission of HCV, complications of HCV as well as the sources of information on knowledge were asked. Information was also obtained on whether they had prior screening of HCV and where the screening was done. The knowledge level score was calculated by computing the number of correct responses to six general questions. Questions assessed respondent’s knowledge on HCV transmission, complications and co-infection. For each correct answer, a score of 1 was allocated, while the score for an incorrect answer was 0. The respondents had good knowledge when they had a score of 3 and above while poor knowledge was equivalent of scores 2 and below [20].
Specimen Collection
About 4 milliliters of venous blood was collected from participants in plain bottles and labeled using individuals’ codes and unique serial numbers from their questionnaires. All safety precautions were observed. The samples were allowed to coagulate and the cloth containing specimens were then centrifuged at the speed of 2,500 revolutions per minute for 5 min. The serum was separated from the cells with micropipette and then put into a properly labeled tiny vials (that contained the individual’s codes and serial numbers) and stored at -8°C in the freezer. The process was repeated on all antenatal clinic days.
Laboratory tests
The anti-HCV screening test was performed on the serum samples using a quick chromatographic immunoassay kit (ACON Hepatitis C Test Strip and DiaSpot HCV One Step). The vials were allowed to reach room temperature before being opened since the test strips needed time to adjust to room temperature before testing. The arrows on the test strip were positioned downward, and it was submerged vertically into the serum for 10 to 15 sec. To avoid crossing it, the maximum line (MAX) on the strip was observed. A ten-minute countdown was begun when the strip was placed on a non-absorbent surface to observe if the red line would form. The intensity of the red hue depends on how much HCV is present in the samples. Hence, any shade of red in the test (T) zone was considered positive. One red line on the control (C) region and no red color at all on the test (T) region were examples of negative result. All specimens that tested positive were confirmed using an ELISA of the third generation in accordance with the manufacturer's instructions (MONOLISA anti-HCV plus version 2, Biorad, Marnes-La-Coquette, France). Test results were swiftly recorded on the corresponding codes and serial numbers to avoid record errors and confusion.
Data analysis
Data was analyzed with the Statistical Package for Social Sciences (SPSS). Frequencies and proportions were obtained through univariate analysis. Bivariate analysis was done to ascertain relationships between the knowledge of respondents/access of respondents to HCV screening and socio-demographic characteristics. Chi square testing was carried out to assess statistical significance which was assumed at 0.05%. Logistic regression analysis was performed to address confounders.
RESULT
Sociodemographic Characteristics of Participants
Table 1 displayed the sociodemographic characteristics of respondents. Four hundred pregnant women were involved in the study. The mean age of participants was 30.6 years and a standard deviation of ± 5.0 years. Most of the study participants 133(33.2%) were within age group 30-34, majority of them 238(59.5%) obtained tertiary education, 396(99.0%) were married, 110(27.5%) were traders and 329(82.3%) were of monogamous family setting.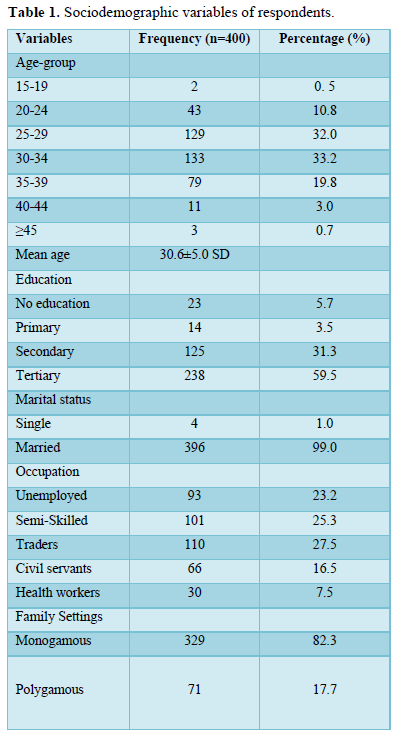
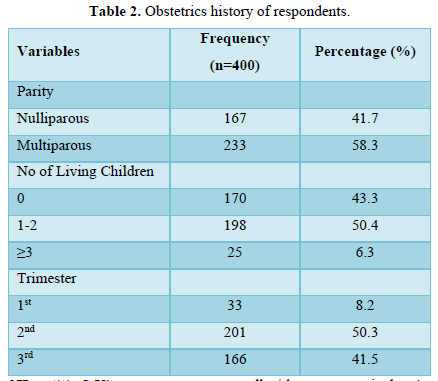
Table 2 displayed the obstetrics history of participants. About 233(58.3%) had more than one prior deliveries (multiparous), 198(50.4%) had 1-2 living children, and 201(50.3%) were in their second trimester.
Respondents’ knowledge of Hepatitis C Virus
Table 3 showed knowledge of respondent on HCV. About 86(46.5%), 159(39.8%), and 174(43.5%) of respondents knew that HCV is transmitted through blood transfusion, unprotected sexual intercourse and contaminated needles/sharps respectively. Again 75(18.8%) knew HCV has no vaccine, 87(21.8%) knew that an individual can be co-infected with HCV and HIV and 63(15.8%) knew that HCV could cause liver cancer.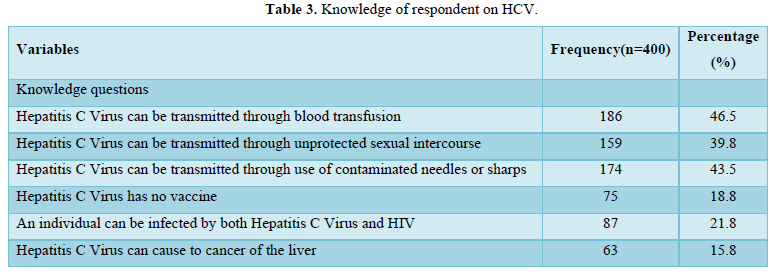
Table 4 showed respondents’ knowledge score on HCV. About 124(31%) of respondents had good knowledge while 276 (69%) had poor knowledge.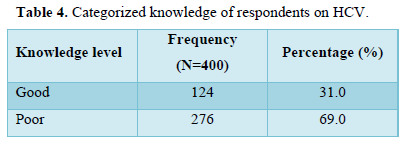
Figure 1 showed the sources of information on HCV among respondents. Almost 88(22.0%) of the respondents reported “asking health workers” as their major sources of information on HCV while others reported “general counseling in health facilities” 29 (7.3%), media 28 (7.0%), personal search 24 (6.0%), internet 22 (5.5%), “discussion with non-health workers” 11 (2.8%), posters in health facilities 8 (2.0%) and posters in non-health facilities 2 (0.5%).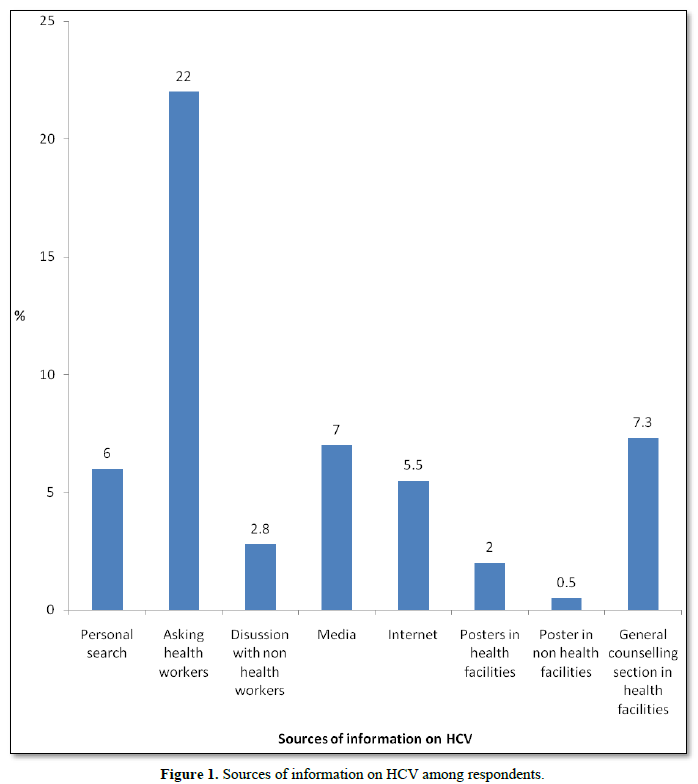
Association between respondent’s knowledge of HCV and socio demographic variables
Table 5 showed association between respondent’s knowledge of HCV and their socio-demographic characteristics. Good knowledge of HCV was significantly associated with education and occupation of respondents (P=0.000).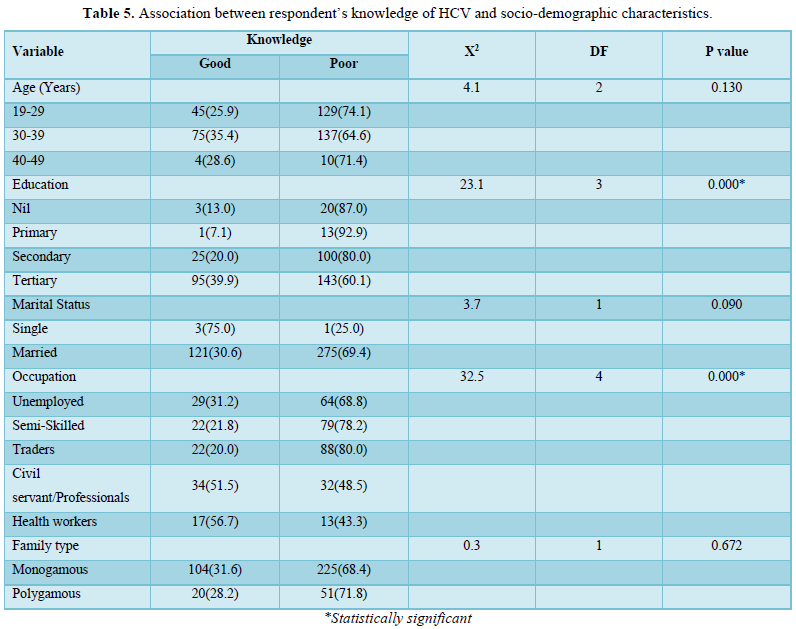
Table 6 showed that there was no significant association between respondent’s knowledge of HCV and their obstetrics history.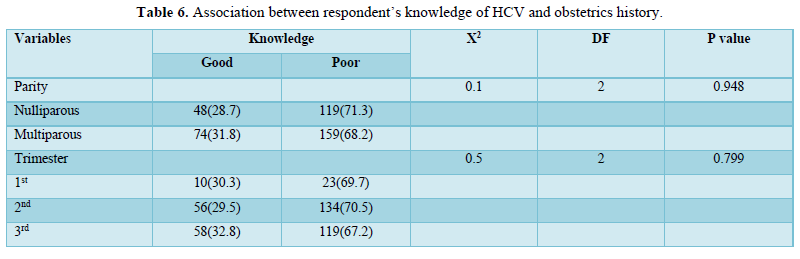
Predictors of knowledge of HCV
Table 7 showed logistic regression model of factors influencing knowledge of respondents on HCV. Being a health worker (OR=. 0.3, CI=0.15-0.81, P=0.014) and a professional/civil servant (OR=. 0.4, CI=0.22-0.82, P=0.010) were found to be significant predictors of good knowledge of HCV. Also, respondents who had tertiary education are 3.1 times more likely to have good knowledge of HCV that those with primary and secondary education although this was not statistically significant.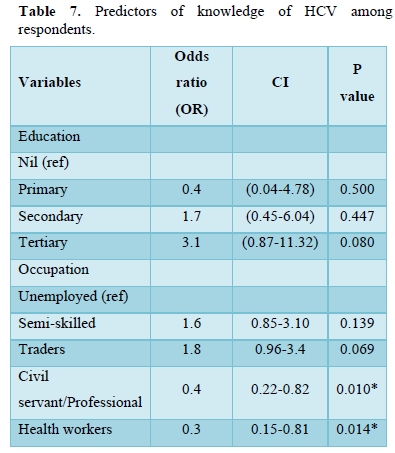
Sero-prevalence of HCV among respondents
Table 8 showed seroprevalence of HCV antibodies among respondents. Six (6) of the participants were positive for HCV, indicating a seroprevalence of 1.5%.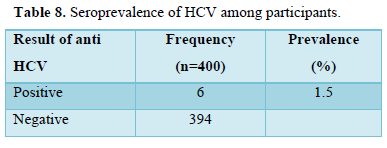
Table 9 showed that among the multiparous pregnant women, the sero-prevalence was 1.25% while it was 0.25% among the nulliparous women.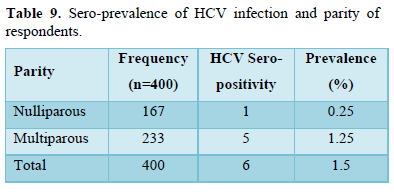
Screening profile for HCV infection among respondents
Table 10 showed that only 39 (9.7%) had previously accessed HCV screening services of which 1(2.6%) was positive for HCV antibodies. Also, only 21(5.3 %) of the study subject were screened in their index pregnancy.
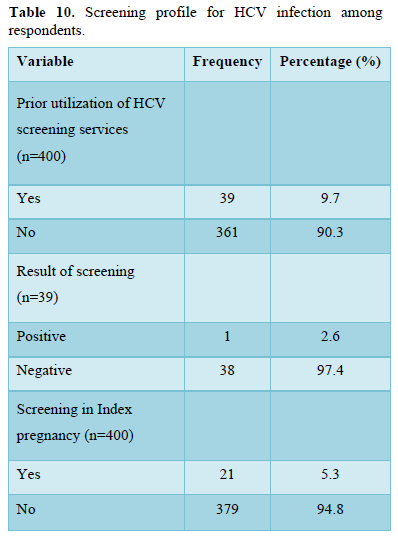
Figure 2 showed where/place of prior utilization of HCV screening services among respondent. Only 39 respondents had previously being screened for HCV out of which 59.0% were screened during antenatal care, 18.0% during pre-employment screening/admission, 17.8% during routine medical checkup, 2.6% during investigation when ill and during blood donation each.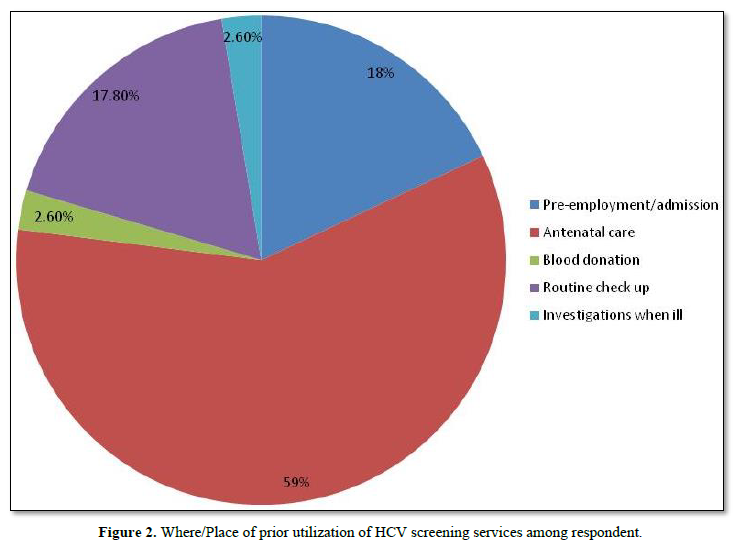
DISCUSSION
This study aimed to assess the level of knowledge about HCV, the prevalence, and the access/utilization of HCV screening among pregnant women attending antenatal clinics in Lagos state, Nigeria. It was noted from the study that there was generally poor knowledge about HCV among the participants. Only 31% of participants had good knowledge as against 69% that had poor knowledge. It was also noted that good knowledge of HCV was significantly associated with education and occupation of respondents. Also, respondents who had tertiary education are more likely to have good knowledge of HCV that those with primary and secondary education. Being a ‘health worker’ and a ‘professional/civil servant’ were found to be significant predictors of good knowledge of HCV. The sero-prevalence of HCV antibodies among respondents was 1.5% with higher prevalence among multiparous compared to nulliparous women. The study also showed low level of access to screening. Only 9.7% of the participants had previously accessed HCV screening services. Also, just about 5.3 % of the study subject were screened in their index pregnancy, however amongst the few of them screened, antenatal clinics remains the place where greater number of them had access to HCV screening.
Evaluation of knowledge of HCV infection in this study showed that up to 69% of the participants had poor knowledge of hepatitis C virus infection with only about 31% of the participants being aware of the infection. This indicated a knowledge gap among the women. This finding is similar to that of Achinge [21] in Markurdi, North Central Nigeria where 76% of the respondent had poor knowledge of HCV infection [21]. It is also similar to the findings in a nationwide Nigerian pilot cross sectional study by Eleje [22], where greater than 50 % of the participants were not aware of HCV infection [22]. However, this result is in contrast with that of the study carried out in the United States of America amongst expectant mothers who suffered opioid consumption disorder [23]. It was noted in their study that almost 90% of the women were aware of hepatitis C infection. The differential findings in these studies may be due the difference in epidemiology of HCV infection in the different settings/ populations where the studies were carried out. Again, while this current study was among antenatal attendees, the study in the United States was done among women with opioid use disorder who may have been exposed to information on risk factors of HCV infection by their health care providers even before the study. It was also noted that having a tertiary education and being a health worker or a professional civil servant is significantly associated with good knowledge and awareness of HCV infection. This finding is similar to that found by Eleje [22] in Nigeria and in Ethiopia among women attending antenatal care [24,25]. The good knowledge as found in this subset may reflect a greater exposure to information about HCV due to the nature of their jobs and probably larger income which could be used to access information.
The poor knowledge of HCV infection among the participants in this study is bothersome and therefore there is the need for a more effective health education on epidemiology of HCV infection among the pregnant women. In this study it was also noted that antenatal clinics remained the place where greater number of women had access to HCV screening. The antenatal care offers a huge opportunity to give health education to pregnant women about infectious diseases including HCV infection.
The source of information among the participants was mainly through asking health workers (22%), followed by counseling section at the health care facilities (7.3%) and media (7.0%). This emphasizes the importance of information given at our health care facilities. The programme should be enriched and well packaged to make attendees adequately educated about public health issues such as hepatitis C virus infection. Education of patients and health providers about Hepatitis C virus infection has been noted to be useful in improving knowledge levels [26]. Hence, it may be reasonable to expect that increased awareness and communication strategies by the mass media may aid in increasing knowledge of HCV.
The sero-prevalence of HCV antibodies among respondents was 1.5% with higher prevalence among multiparous compared to nulliparous women. This finding is similar with prevalence of 1.3% noted in a nationwide study in Nigeria [22], 1.86% found among 269 booked pregnant women attending antenatal care in Benin City, Edo-State [2], 1.39% noted among pregnant women at a tertiary institution in Ekiti State [7] and 1.1% found among 267 pregnant women attending Igbinedion University Teaching Hospital Okada [28]. However, this prevalence was higher than 0.5% found in Nigeria [29] and 0.3% found among Indian antenatal attendees [30].
The study also showed low level of access to screening. Only 9.7% of the participants had previously accessed HCV screening services with just about 5.3 % of the study subject screened in their index pregnancy. This showed that uptake of screening services is really low suggesting that targeted or risk-based approach to HCV screening in pregnancy as commonly practiced in Nigeria is poor. Quite unlike testing for HIV or hepatitis B infection, HCV testing for pregnant women is not routinely practiced but restricted to at risk group and as such most pregnant women infected with HCV may go unidentified. The risk based or the targeted approach to screening has repeatedly been shown to miss most cases of HCV infection [31]. This may be due to health providers’ inability to ask about risk factors or that patients do not voluntarily give out this information even when asked. Women especially the young ones persistently seek for medical care when they are pregnant [32]. They have high compliance for testing as well as follow up because of the consideration given to the future of their unborn babies [33], however the compliance and the zeal to follow up treatment wanes after delivery [32]. This underscores the need for universal screening of these women using the opportunity offered by pregnancy and antenatal attendance. Offering such routine screening as done with other infectious diseases in pregnancy may influence patient behavior and health care provider decision and in effect help reduce maternal to child transmission of HCV infection. Again, if the transmission does occur, the awareness of the HCV status may help in facilitating proper evaluation of the HCV exposed babies. As noted in this study, the low access to HCV screening even among the participants who presented for antenatal care highlights the need to utilize the opportunity offered by antenatal care to educate women about HCV infection, associated risk factors and modes of transmission as well as the complications and then offer routine screening.
CONCLUSION
There was poor knowledge of HCV infection among the pregnant women in Lagos, Nigeria. Only about 31% of the participants have good knowledge and this is positively affected by tertiary level of education and occupation of the respondents. The prevalence of HCV infection was 1.5%. There was also low level of access to screening even among those who presented for antenatal care, however antenatal clinics remains the place where greater number of those screened had access to HCV screening. Considering the socioeconomic and health burden due to Hepatitis C virus infection in Nigeria, there is the need for a policy change towards routine screening of pregnant women.
LIMITATIONS
This study was a single center study with relatively small sample size and limited geographical spread and therefore the findings cannot be generalized to whole Nigerian population. However, a larger, multicenter study is needed to further validate this finding and clarify the impact of routine screening on the epidemiology of HCV infection in Nigeria.
- Programmes STI (2012) Prevention and Control of Viral Hepatitis Infection: Framework for Global Action. Who.int. World Health Organization.
- Blach S, Zeuzem S, Manns M, Altraif I, Duberg A-S, et al. (2017) Global prevalence and genotype distribution of hepatitis C virus infection in 2015: A modeling study. Lancet Gastroenterol Hepatol 2(3): 161-176.
- Averhoff FM, Glass N, Holtzman D (2012) Global burden of hepatitis C: Considerations for healthcare providers in the United States. Clin Infect Dis 55(1): S10-S15.
- World Health Organization (2023) Hepatitis C. Available online at: https://www.who.int/news-room/fact-sheets/detail/hepatitis-c
- World Health Organization (2023) Global policy report on the prevention and control of viral hepatitis in WHO member states. Available online at: https://apps.who.int/iris/bitstream/handle/10665/85397/9789241564632_eng.pdf
- Arshad M, El-Kamary SS, Jhaveri R (2011) Hepatitis C virus infection during pregnancy and the newborn period - are they opportunities for treatment? J Viral Hepat 18(4): 229-236.
- Esan AJ, Omisakin CT, Ojo-Bola T, Owoseni MF, Fasakin KA, et al. (2014) Sero-prevalence of hepatitis B and hepatitis C virue co-infection among pregnant women in Nigeria. Am J Biomed Res 2(1): 11-15.
- Ugbebor O, Aigbirior M, Osazuwa F, Enabudoso E, Zabayo O (2011) The prevalence of hepatitis B and C viral infections among pregnant women. N Am J Med Sci 3(5): 238-241.
- Reddick KLB, Jhaveri R, Gandhi M, James AH, Swamy GK (2011) Pregnancy outcomes associated with viral hepatitis: Hepatitis and pregnancy. J Viral Hepat 18(7): e394-e398.
- Safir A, Levy A, Sikuler E, Sheiner E (2010) Maternal hepatitis B virus or hepatitis C virus carrier status as an independent risk factor for adverse perinatal outcome. Liver Int 30(5): 765-770.
- gov. [cited 2023 Mar 8].
- Mitchell AE, Colvin HM. Palmer Beasley (2010) R: Institute of Medicine recommendations for the prevention and control of hepatitis B and C. Hepatology 51(3):729–733.
- Hagan H, Campbell J, Thiede H, Strathdee S, Ouellet L, et al. (2006) Self-reported hepatitis C virus antibody status and risk behavior in young injectors. Public Health Rep 121(6): 710-719.
- http://file:///C:/Users/anayo/Desktop/hepatitis%20c%20study/Background%20and%20Rationale%20for%20Hepatitis%20C.htm
- Mboto CI, Ebenge IA, Ogban IE, Andrew PJ (2010) Prevalence, Sociodemographic Characteristics and Risk Factors for Hepatitis C Infection among Pregnant Women in Calabar Municipality. Vol. 10. Nigeria Hepat Mon. Spring.
- Hepatitis B Foundation (2020) The Journey to Hepatitis Elimination in Nigeria [Internet].
- Filani MO (2012) The Changing Face of Lagos: From Vision to Reform and Transformation pp: 8-11.
- Sotunde OF, Sanni SA, Onabanjo OO, Olayiwola IO, Agbonlahor M (2014) A retrospective study of the health profile of neonates of mothers with anemia in pregnancy and pregnancy induced hypertension in Lagos, Nigeria. J Public Health Africa 5(2): 286.
- Ogunro PS, Adekanle DA, Fadero FF, Ogungbamigbe TO, Oninla SO (2007) Prevalence of anti-hepatitis C virus antibodies in pregnant women and their offspring in a tertiary hospital in Southwestern Nigeria. J Infect Dev Ctries 1(3): 333-336.
- Adeyemi AB, Enabor OO, Ugwu IA, Bello FA, Olayemi OO (2013) Knowledge of hepatitis B virus infection, access to screening and vaccination among pregnant women in Ibadan, Nigeria. J Obstet Gynaecol 33(2): 155-159.
- Achinge G, Malu A, Mbaave P, Bitto T, Shaahu V, et al. (2013) Prevalence of Hepatitis C in Makurdi, North Central Nigeria. North Central Nigeria. IOSR J Dent Med Sci 7(5): 6-10.
- Eleje GU, Rabiu A, Mbachu II, Akaba GO, Loto OM, et al. (2021) Awareness and prevalence of hepatitis C virus infection among pregnant women in Nigeria: A national pilot cross-sectional study. Res Square 17: 17455065211031718.
- Krans EE, Rothenberger SD, Morrison PK, Park SY, Klocke LC, et al. (2018) Hepatitis C virus knowledge among pregnant women with opioid use disorder. Matern Child Health J 22(8): 1208-1216.
- Dabsu R, Ejeta E (2018) Seroepidemiology of hepatitis B and C virus infections among pregnant women attending antenatal clinic in selected health facilities in East Wollega Zone, West Oromia, Ethiopia. Biomed Res Int 2018: 4792584.
- Dagnew M, Million Y, Gizachew M, Eshetie S, Yitayew G, et al. (2020) Hepatitis B and C viruses’ infection and associated factors among pregnant women attending antenatal care in hospitals in the Amhara National Regional State, Ethiopia. Int J Microbiol 2020: 8848561.
- Giles ML, Garland SM, Grover SR, Lewin SM, Hellard ME (2006) Impact of an education campaign on management in pregnancy of women infected with a blood‐borne virus. Med J Aust 184(8): 389-392.
- Onakewhor J, Okonofua F (2009) Seroprevalence of hepatitis c viral antidodies in pregnancy in a tertiary health facility in Nigeria. Niger J Clin Pract 12: 65-73.
- Oladeinde BH, Omoregie R, Olley M, Anunibe JA, Oladeinde O (2012) Hepatitis B and C Viral Infections among Pregnant Women in a Rural Community of Nigeria. Int J Basic Appl Virol 1(1): 1-5.
- Omatola CA, Okolo MO, Abraham JO (2018) Hepatitis C virus co infection in human immunodeficiency virus infected pregnant women in Anyigba, Kogi State, Nigeria. Nat Sci 16: 62-68.
- Malhotra P, Nanda S, Malhotra V (2016) Prevalence of HIV, hepatitis B, hepatitis C in pregnancy at tertiary care center of Northern India. Adv Res Gastroentero Hepatol 1(4): 80-82.
- El-Kamary SS, Hashem M, Saleh DA, Ehab M, Sharaf SA, et al. (2015) Reliability of risk-based screening for hepatitis C virus infection among pregnant women in Egypt. J Infect 70(5): 512-519.
- Jhaveri R, Broder T, Bhattacharya D, Peters MG, Kim AY, et al. (2018) Universal screening of pregnant women for hepatitis C: The time is now. Clin Infect Dis 67(10): 1493-1497.
- Mellins CA, Chu C, Malee K, Allison S, Smith R, et al. (2008) Adherence to antiretroviral treatment among pregnant and postpartum HIV-infected women. AIDS Care 20(8): 958-968.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)




