Mini-Review
Quorum Sensing Inhibitor: A New Strategy Against Pathogenic Bacteria
5580
Views & Citations4580
Likes & Shares
Due to the increasing resistance of antibiotics from the last few decades, it is crucial to find new techniques against pathogenic bacteria. Quorum sensing (QS) is the alternative target to introduce a new strategy. The gene expression of QS has occurred in a cell density-dependent manner. Synthetic and natural QS inhibitors (QSIs) play a crucial role to inhibit these QS signals. In this review, we highlight some examples of natural and synthetic QSIs. This short review also focuses on the inhibition of QS mechanisms with some of its applications.
Keywords: Quorum sensing, Pathogenic bacteria, QS inhibitors
INTRODUCTION
The insecure and excessive use of antibiotics causes several problems like the emergence of multi-drug resistance of pathogenic bacteria and thus becomes a serious problem to the health system of human and domestic animals [1,2]. A large number of infectious diseases are linked to the enormous growth of bacterial biofilm formation [3]. Quorum sensing (QS) controls this bacterial behavior by the secretion of signal molecules in a cell density-dependent manner [4,5]. So, QS is gaining now as an important therapeutic target because QS inhibitory drugs have more specific effects than traditional antibiotics [6]. Quorum sensing inhibitor (QSI) has been widely studied in recent times due to its feasibility and applicability in combating pathogens. Generally, two types of QSIs are found. One type contains small molecule QSIs, extracted from either natural resources or obtained from chemical synthesis [7,8]. The other type contains quorum quenching (QQ) enzymes includes acyl-homoserine lactones (AHLs) and autoinducer-2 (AI-2) kinases as signaling molecules [9-11].
QUORUM SENSING SIGNALS
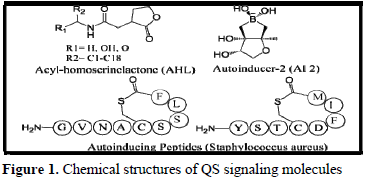
Acyl-homoserinelactones (AHLs), autoinducing peptides (AIPs) and autoinducer-2 (AI-2) (Figure 1) are mainly found in forming bacterial QS signals by the formation of biofilm, conjugation of plasmid and finally affect the antibiotic resistance due to survival capacity of bacteria from difficulty in the environment [12]. The process of QS signaling cell to cell communications is fully different for both Gram-positive and Gram-negative bacteria. Gram-negative bacteria mainly produce AHL signaling molecules whereas, Gram-positive bacteria produce AIP signaling molecules but AI-2 signals are produced from both of these two bacteria [13-15] (Figure 2).
Characteristic of QSI
Firstly, an ideal QSI has a low molecular weight which significantly reduces the expression of QS controlled genes. Secondly, QSI has shown a higher degree of specificity for the QS regulator (LuxR homologue). Thirdly, this QSI should be chemically stable with metabolic resistance and disposed of by a higher host organism [16].
Quorum sensing inhibition mechanism
The working principles of most of the QSIs are based on the following schemes [17].
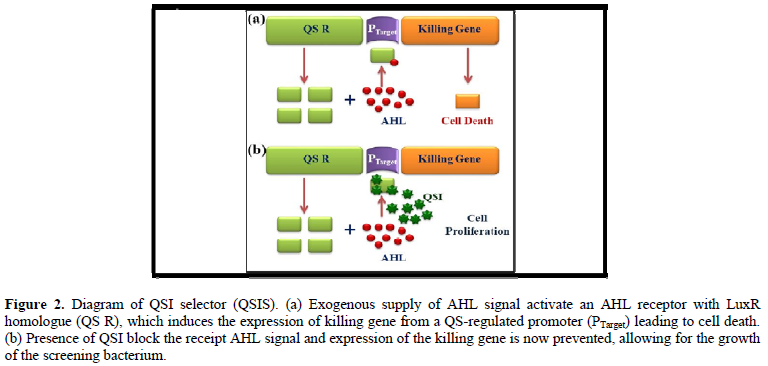
Degrading QS signals
Degradation of the QS signal can happen either by enzymatically or nonenzymatically. Generally, three types of enzymes are responsible to target AHL signals. AHL lactonase hydrolyzes ester bond (homoserine lactone ring) of AHL [6,18]. Acylase is another enzyme that degrades the AHL signal and forms homoserine lactone (HSL) and 3-oxodecanoic acid as major products [19]. Oxido-reductase is the third enzyme, responsible for QS signal degradation by degrading AHL [20].
Biosynthesis of inhibited QS signal
The suppression of AHL production can be occurred theoretically by hampering S-adenosylmethionine (SAM) biosynthesis or by inactivating synthase enzyme [6]. Some QSIs are generated by targeting LuxS. QSI activity to inhibit LuxS was first found by S-anhydroribosyl-L-homocysteine and S-homoribosyl-L-cysteine [21]. Recently, some potent small molecules are found that quenched S. mutans QS by inhibiting the peptidase activity of the ComA cassette [22].
Detected QS signal inhibition
Detected QS signal can be inhibited by altering downstream signals of non-productive signal-receptor complexes. Computer-aided structural modification of known inhibitors is another possibility to improve QS inhibitory activity [6]. Some researchers also found non-AHL based pharmacophores that inhibit LuxR proteins [23,24].
Antibiotics as QS inhibitors
There are many shreds of evidence for the use of antibiotics that target QS. Antibiotics such as azithromycin, ceftazidime, and ciprofloxacin also have QSI activity [6]. Zosteric acid (phenolic compound) is another compound that has inhibitory activity against Candida albicans [25]. Similarly, ursolic acid suppresses the biofilm formation of E. coli, P. aeruginosa, Vibrio harveyi [26].
EXAMPLE OF QSIS
Natural quorum sensing inhibitors
Due to the co-existence of various plants and fungi with QS bacteria, they have evolved natural QSIs to reduce bacterial infection. Cyclic Sulphur compounds, halogenated furanones and penicillanic acid belong to this category [27-29].
Plant-based QSI
Garlic extracts with 4-nitropyridine-N-oxide have an inhibitory activity of QS activated virulence genes [30]. Chloroform and methanol extract of clove also have inhibitory activity towards QS signaling in E. coli [31]. Γ-amino butyric acid (GABA) produced form plants have promoted the AHL signal degradation by lactonase [32,33]. Pyrogallol came from Emblica Officinalis have antagonism activity against AI-2 [34]. Curcumin from Curcuma longa reduces the virulence genes expression of P. aeruginosa [35]. Furocoumarins, limonoids, and cinnamaldehyde derivatives also have QSI abilities [36,37]. Flavonoids such as kaempferol, apigenin, naringenin, and quercetin have various QSI activities [38].
Fungus based QSIs
Antibiotics are produced as secondary metabolites from fungi. Since penicillin has quite an activity to control bacterial infections and thus act as QSI (1). Auricularia auricular created natural pigments which have QSI activity to inhibit violacein production in C. violaceum [39].
Marine organism-based QSIs
Delisea pulchra produced halogenated furanones which inhibit bacterial AHL signals of QS mediated activity. Cyanobacteria (marine organisms) also inhibit QS gene expression. Lyngbyoic acid and malyngolide (isolate from Lyngbya majuscule) also inhibit violacein production in C. violaceum and pyocyanin elastase production in P. aeruginosa [40,41]. In P. areuginosa, Malyngamide-C, and 8-epi-malygamide (extracted from L. majuscule) are also developed as QSI [42].
Synthetic quorum sensing inhibitors
Substitution of C-3 atom by Sulphur in the acyl side chain of AHL created compounds that effectively block QS expression in both LasR and LuxR. Similarly, aryl substitution at the end of the side chain produced other successful QSIs [43]. The QSI potency of aryl AHL can be further increased by substituting the carbonyl group (C-1) of the side chain with the sulphonyl group [44].
APPLICATION OF QSIS
QSI has a wide range of applications in various fields like the human health system, food industry. Hentzer et al. suggested that QSI can effectively reduce biofouling of surgical implants (caused by P. aeruginosa) on the surface device [45]. QS signal of Vibrio cholera is targeted by some QSI for developing cholera therapy [46]. The combined form of antibiotics with anti QS strategies can also develop some QSIs for medicinal treatment [6]. Endophytic QS inhibitors owing to biodegradable are more suitable for the food preservation industry [47].
CONCLUSION
In conclusion, QSI gives a new approach with promising activity in the battle against antibiotic-resistant pathogenic bacteria. Hence, combine the effect of QSI with antibiotics may be useful in clinical treatment. Though QSI has a wide range of applications in the enormous field, its production at a large scale is still a matter of great concern. In the future, we hope that more types of QSI will come as safe and suitable antimicrobial drugs.
- Kalia VC, Rani A, Lal S, Cheema S, Raut CP (2007) Combing databases reveal potential antibiotic producers. Expert Opin Drug Discov 2: 11-24.
- Radic N, Strukelj B (2012) Endophytic fungi: The treasure chest of antibacterial substances. Phytomedicine 19: 1270-1284.
- Lewis K (2007) Persister cells, dormancy, and infectious disease. Nat Rev Microbiol 5: 48-56.
- Miller MB, Bassler BL (2001) Quorum sensing in bacteria. Annu. Rev. Microbiol. 55: 165-199.
- Jayaraman A, Wood TK (2008) Bacterial quorum sensing: Signals, circuits, and implications for biofilms and disease. Ann Rev Biomed Eng 10: 145-167.
- LaSarre B, Federle MJ (2013) Exploiting quorum sensing to confuse bacterial pathogens. Microbiol Mol Biol Rev 77: 73-111.
- Kalia VC (2013) Quorum sensing inhibitors: An overview. Biotechnol Adv 31: 224-245.
- Defoirdt T, Brackman G, Coenye T (2013) Quorum sensing inhibitors: How strong is the evidence? Trends Microbiol. 21: 619-624.
- Dong YH, Zhang LH (2005) Quorum sensing, and quorum-quenching enzymes. J Microbiol 43: 101-109.
- Czajkowski R, Jafra S (2009) Quenching of acyl-homoserine lactone-dependent quorum sensing by enzymatic disruption of signal molecules. Acta Biochimica Polonica 56: 1-16.
- Fetzner S (2015) Quorum quenching enzymes. J Biotechnol 201: 2-14.
- Eickhoff MJ, Bassler BL (2018) Snapshot: Bacterial quorum sensing Cell 174(5): 1328.
- Schuster M, Sexton DJ, Diggle SP, Peter E (2013) Acyl-homoserine lactone quorum sensing: from evolution to application. Ann Rev Microbiol 67: 43-63.
- Sturme MH, Kleerebezem MK, Nakayama J, Akkermans AD, Vaugha EE, et al. (2002) Cell to cell communication by autoinducing peptides in gram-positive bacteria. Antonie Van Leeuwenhoek. 81: 233-243.
- Pereira CS, Thompson JA, Xavier KB (2013) AI-2-mediated signalling in bacteria. FEMS Microbiol Rev 37: 156-181.
- Rasmussen TB, Givskov M (2006) Quorum sensing inhibitors: A bargain of effects. Microbiol 152: 895-904.
- Sarkar K, Das RK (2019) A review on quorum sensing inhibitors. IJPSR. 10 (12): 5224-5233.
- Dong YH, Wang LH, Xu JL, Zhang HB, Zhang XF, et al. (2001) Quenching quorum sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature 411: 813-817.
- Lin YH, Xu JL, Hu J, Wang LH, Ong SL, et al. (2003) Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorumquenching enzymes. Mol Microbiol 47: 849-860.
- Bijtenhoorn P, Schipper C, Hornung C, Quitschau M, Grond S, et al. (2011) BpiB05: A novel metagenome-derived hydrolase acting on N-acyl homoserine lactones. J Biotechnol 155: 86-94.
- Alfaro JF, Zhang T, Wynn DP, Karschner EL, Zhou ZS (2004) Synthesis of LuxS inhibitors targeting bacterial cell-cell communication. Org Lett 6: 3043-3046.
- Ishii S, Fukui K, Yokoshima S, Kumagai K, Beniyama Y, et al. (2017) High-throughput screening of small molecule inhibitors of the Streptococcus quorum-sensing signal pathway. Sci Rep 7: 4029.
- Ishida T, Ikeda T, Takiguchi N, Kuroda A, Ohtake H, et al. (2007) Inhibition of quorum sensing in Pseudomonas aeruginosa by N-acyl cyclopentylamides. Appl Environ Microbiol 73: 3183-3188.
- Soulere L, Sabbah M, Fontaine F, Queneau Y, Doutheau A (2010) LuxR-dependent quorum sensing: computer-aided discovery of new inhibitors structurally unrelated to Nacylhomoserinelactones. Bioorg Med Chem Lett 20: 4355-4358.
- Villa F, Cappitelli F (2013) Plant-derived bioactive compounds at sub-lethal concentrations: towards smart biocide-free antibiofilm strategies. Phytochem Rev 12: 245-254.
- Ren D, Zuo R, Gonzalez Barrios AF, Bedzyk LA, Eldridge GR, et al. (2005) Differential gene expression for investigation of Escherichia coli biofilm inhibition by plant extract ursolic acid. Appl Environ Microbiol 71: 4022-4034.
- Persson T, Hansen TH, Rasmussen TB, Skindersø MB, Givskov M, et al. (2005) Rational design and synthesis of new quorum-sensing inhibitors derived from acylated homoserine lactones and natural products from garlic, Org. Biomol.Chem 3 (2): 253-262.
- Givskov M, De Nys R, Manefield M, Gram L, Maximilien R, et al. (1996) Eukaryotic interference with homoserine lactone-mediated prokaryotic signaling. J Bacteriol 178 (22): 6618-6622.
- Rasmussen TB, Skindersoe ME, Bjarnsholt T, Phipps RK, Christensen KB, et al. (2005) Identity and effects of quorum-sensing inhibitors produced by Penicillium species, Microbiology 151 (5): 1325-1340.
- Rasmussen TB, Bjarnsholt T, Skindersoe ME, Hentzer M, Kristoffersen P, et al. (2005) Screening for quorum sensing inhibitors (QSI) by use of a novel genetic system, the QSI selector, J. Bacteriol. 187 (5): 1799-1814.
- Krishnan T, Yin WF, Chan KG (2012) Inhibition of quorum sensing-controlled virulence factor production in Pseudomonas aeruginosa PAO1 by Ayurveda spice clove (Syzygium aromaticum) bud extract, Sensors 12 (4): 4016-4030.
- Chevrot R, Rosen R, Haudecoeur E, Cirou A, Shelp BJ, et al. (2006) GABA controls the level of the quorum-sensing signal in Agrobacterium tumefaciens. Proc Natl Acad Sci U S A 103: 7460-7464.
- Zhang HB, Wang LH, Zhang LH (2002) Genetic control of quorum-sensing signal turnover in Agrobacterium tumefaciens. Proc Natl Acad Sci 99: 4638-4643.
- Ni N, Choudhary G, Li M, Wang B (2008) Pyrogallol and its analogs can antagonize bacterial quorum sensing in Vibrio harveyi. Bioorg Med Chem Lett 18: 1567-1572.
- Rudrappa T, Bais HP (2008) Curcumin, a known phenolic from Curcuma longa, attenuates the virulence of Pseudomonas aeruginosa PAO1 in the whole plant and animal pathogenicity models. J Agric Food Chem 56: 1955-1962.
- Lönn-Stensrud J, Petersen FC, Benneche T, Scheie AA (2007) Synthetic bromated furanone inhibits autoinducer-2-mediated communication and biofilm formation in oral streptococci. Oral Microbiol Immunol 22: 340-346.
- Brackman G, Defoirdt T, Miyamoto C, Bossier P, Van Calenbergh S, et al. (2008) Cinnamaldehyde and cinnamaldehyde derivatives reduce virulence in Vibrio spp.by decreasing the DNA-binding activity of the quorum sensing response regulator LuxR. BMC Microbiol 8: 149.
- Vikram A, Jayaprakasha GK, Jesudhasan PR, Pillai SD, Patil BS (2010) Suppression of bacterial cell-cell signaling, biofilm formation, and type III secretion system by citrus flavonoids Appl Microbiol 109: 515-527.
- Zhu H, He C-C, Chu QH (2011) Inhibition of quorum sensing in Chromobacterium violaceum by pigments extracted from Auricularia auricular. Lett Appl Microbiol 52: 269-274.
- Dobretsov S, Teplitski M, Alagely A, Gunasekara SP, Paul VJ (2010) Malyngolide from the cyanobacteriumLyngbya majuscula interferes with quorum sensing circuitry. Environ Microbiol Rep 2: 739-744.
- Kwan JC, Meickle T, Ladwa D, Teplitski M, Paul V, et al. (2011) Lyngbyoic acid, a "tagged" fatty acid from a marine cyanobacterium, disrupts quorum sensing in Pseudomonas aeruginosa. Mol Biosyst 7: 1205-1216.
- Kwan JC, Teplitski M, Gunasekara SP, Paul VJ, Luesch H (2010) Isolation and biological evaluation of 8-epi-malyngamide C from the Floridian marine cyanobacterium Lyngbyamajuscula. J Nat Prod 73: 463-466.
- Reverchon S, Chantegrel B, Deshayes C, Doutheau A, Cotte-Pattat N (2002) New synthetic analogues of N-acyl homoserinelactones as agonists or antagonists of transcriptional regulators involved in bacterial quorum sensing. Bioorg Med Chem Lett 12: 1153-1157.
- Castang S, Chantegrel B, Deshayes C, Dolmazon R, Gouet P, et al. (2004) N-Sulfonyl homoserine lactones as antagonists of bacterial quorum sensing. Bioorg Med Chem Lett 14: 5145-5149.
- Hentzer M, Riedel K, Rasmussen TB, Heydorn A, Andersen JB, et al. (2002) Inhibition of quorum sensing in Pseudomonas aeruginosa biofilm bacteria by a halogenated furanone compound. Microbiology 148: 87-102.
- Jude BA, Martinez RM, Skorupski K, Taylor RK (2009) Levels of the secreted Vibrio cholera attachment factor GbpA are modulated by quorum-sensing-induced proteolysis. J Bacterial 191: 6911-6917.
- Nithya V, Murthy PS, Halami PM (2013) Development and application of active films for food packaging using antibacterial peptide of Bacillus licheniformis Me1. J Appl Microbiol 115:475-483.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Allergy Research (ISSN:2642-326X)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Pathology and Toxicology Research
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)




