Research Article
Effect of Biofield Energy Healing-Based Proprietary Test Formulation on Stress Hormones Using Unpredictable Chronic Stress (UCS) Model in Sprague Dawley Rats
9984
Views & Citations8984
Likes & Shares
The present study was designed to evaluate the impact of Consciousness Energy Healing Treatment (the Trivedi Effect®) on a novel test formulation in male Sprague Dawley (SD) rats using unpredictable chronic stress (UCS) model for the estimation of stress hormones using ELISA assay. A test formulation was formulated including minerals (magnesium, zinc, copper, calcium, selenium, and iron), vitamins (ascorbic acid, pyridoxine HCl, alpha tocopherol, cyanocobalamin, and cholecalciferol), Panax ginseng extract, β-carotene, and cannabidiol isolate. The constituents of test formulation were divided into two parts; one section was defined as the untreated test formulation, while the other portion of the test formulation and three group of animals received Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The plasma corticosterone level data showed that the level was significantly reduced by 49.8%, 75.2% (p≤0.05), 41.8%, 47.9% and 28.7% in the Biofield Energy Treated Test formulation to the untreated rats (G5), Biofield Energy Treatment per se to the rats (G6), 15 days pre-treatment of Biofield Energy Treated Test formulation (G7), 15 days pre-treatment of Biofield Energy Treated Test formulation to the Biofield Energy Treated rats (G8), and Untreated Test formulation to the Biofield Energy Treated per se rats (G9) groups, respectively as compared with the untreated test formulation group (G4). The level of plasma angiotensin-II was significantly decreased by 40.6% (p≤0.001), 46% (p≤0.001), 35.9% (p≤0.001), 21.5%, and 35.5% (p≤0.001) in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G4. The level of plasma noradrenaline after treatment was reduced by 13.6%, 20.2%, and 28.4% in the G6, G7, and G8 groups, respectively compared to the G4. In addition, plasma epinephrine level after treatment was significantly reduced by 51.9% (p≤0.05), 51.9% (p≤0.05), 37.7%, 25.6%, and 35.4% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G2 group. Similarly, the level of norepinephrine was measured in CSF, which was significantly decreased by 13.27%, 32.2% (p≤0.05), 19.7%, 13.7%, and 11.5% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G2 group. Overall, the data suggested significance effect of Biofield Energy per se along with preventive measure on the animal with respect to various stress-related disorders. The results showed a significant slowdown of disease progression and all other disease-related complications/symptoms in the preventive Biofield Energy Treatment group per se and the Biofield Energy Treated Test formulation groups (viz. G6, G7, G8, and G9) as compared to the disease control and untreated test formulation groups.
Keywords: Biofield treatment, Stress hormones, The Trivedi Effect®, Unpredictable Chronic Stress, ELISA INTRODUCTION
Psychiatric disorders are known as a major threat in the healthcare system in United States and throughout the world as it poses a significant risk to human health. The research studies reported that in the United States, ~71% of the major depressive disorder is observed with the generalized anxiety disorder in comorbid state; however, the basis for this association is still to be determined [1]. Moreover, the stressful life events could be taken as a common risk factor associated with such disorders [2]. The normal human physiology involves the activation of the hypothalamus-
pituitary-adrenal (HPA) axis that resulted in the glucocorticoid hormones release i.e., cortisol and/or corticosterone. However, the repeated exposure to stress may lead to an excessive activation of HPA axis, which further resulted in the overproduction of glucocorticoids (GCs) [3]. As a consequence, there might be some neuro-chemical and neuro-anatomical alterations occurred in several brain regions such as, the hippocampus, amygdale, prefrontal cortex [4], nucleus accumbens [5], dorsal striatum [6], bed nucleus of the stria terminalis [7] and brain stem [8], etc. Moreover, angiotensin-II (Ang-II) is another important stress hormone. The several research studies reported that in both acutely and chronically stressed animals, the circulating as well as tissue Ang-II are observed to be significantly increased. Besides, the scientists also reported that in humans, the plasma Ang-II content increases markedly after sprinting, as similar to the cortisol that indicated its important role in the stress related disorders [9]. Also, the alteration in the concentration of angiotensin-II and cAMP in plasma, adrenal gland, brain and cardiovascular tissue were significantly studies in rats during the acute and chronic stress. After the studies, the scientists suggested the important role of the circulating and tissue angiotensin-II in the acute and chronic stress responses [10].
Besides, the psychological states in human, whether produced by physiologic or environmental stresses, have major link with the hypersecretion of epinephrine, i.e., another adrenal hormone. The research studies suggested that the prolonged chronic stress can increase the synthesis of epinephrine and its secretion within the organs such as, adrenal and/or the brain. Moreover, the stress-induced release of epinephrine may affect the intactness and functions of the hippocampus and thereby, may cause the impairment of learning on a task [11]. Besides, various psychiatric disorders could be represented by chronic stress-induced depression and they pose a threat to human race with its high morbidity rate. It was reported that the pathogenesis of depression involves stress-induced dysregulation of noradrenergic system. Moreover, the lack of monoamine in the brain is considered as the main causative factor behind pathophysiology of major depressive disorder (MDD) [12].
The novel test formulation was studies for the study of stress hormones in presence of Unpredictable Chronic Stress (UCS) - induced stress and sleep disorders in Sprague Dawley rats and test formulation was treated with Biofield Energy Treatment (a Complementary and Alternative Medicine, CAM) by a renowned Biofield Energy Healer. Biofield Energy Healing approach has been reported to be significant useful method against various pathological conditions [13], which is accepted worldwide. National Center for Complementary/Alternative Medicine (NCCAM) suggested CAM as one of the best alternative complementary health treatment approach [14]. CAM has many benefits as compared with the current preferred treatment approach [15]. Biofield Energy Healing as a CAM health care approach in addition to other therapies, medicines and practices such as deep breathing, natural products, Tai Chi, yoga, therapeutic touch, Qi Gong, Johrei, Reiki, polarity therapy, pranic healing, chiropractic manipulation, meditation, massage, homeopathy, progressive relaxation, special diets, relaxation techniques, movement therapy, Pilates, mindfulness, Ayurvedic medicine, traditional Chinese herbs and medicines in biological systems [16,17]. The Trivedi Effect®-Consciousness Energy Healing therapy as a Conventional therapy have been widely accepted worldwide. The Trivedi Effect®-Consciousness Energy Healing Treatment was scientifically reported on various disciplines such as in the materials science [18,19], agriculture science [20], antiaging [21], gut health [22], nutraceuticals [23], pharmaceuticals [24], overall human health and wellness. In this study, the authors sought to study the impact of the Biofield Energy Treatment (the Trivedi Effect®) for the level of stress hormones using UCS - induced stress and sleep disorders in Sprague Dawley rats using ELISA assays.
MATERIAL AND METHODS
- Chemicals and Reagents
Pyridoxine hydrochloride (vitamin B6), calcitriol, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, provit A) were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (alpha-tocopherol), cholecalciferol (vitamin D3), iron (II) sulphate, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Imipramine hydrochloride was purchased from Sigma, USA. For the estimation of stress biomarker hormonal panel, specific ELISA kits were used, which was procured from CUSABIO, USA.
- Study Design
The current experiment was designed to fulfil the study protocol, animals were assigned into nine (9) groups. G1: Normal control; G2: Disease control (UCS: Unpredictable chronic stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + Untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation).
- Maintenance of Animal
Randomly breed fifty-four male Sprague Dawley (SD) rats with body weight ranges from 200 to 300 gm were used in this study. The animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of 6 animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Govt. of India. The test facility is registered (registration no. 64/PO/br/s/99/CPCSEA) for animal experiments with the CPCSEA. The animals were procured using protocol approved by the Animal Ethics Committee (IAEC/41/505) and the husbandry conditions were maintained as per the recommendations of the CPCSEA.
- Consciousness Energy Healing Strategies
Each ingredient of the test formulation was divided into two parts. The test formulation was divided into two parts, one part of the test compound was not received any sort of treatment and were defined as the untreated or control sample. The second part of the test formulation was treated with the Trivedi Effect® - Energy of Consciousness Healing/Blessing Treatment (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under laboratory conditions for ~3 min. Besides, three group of animals also received Biofield Energy Healing Treatment (known as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar laboratory conditions for ~3 min. The Biofield Energy Healer was located in the USA; however, the test formulation was located in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission was done remotely to the samples or animals. The energy transmission was done without touching the samples or animals. After that, the Biofield Energy Treated/Blessed samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer for ~3 min energy treatment, under the same laboratory conditions. The “sham” healer not has any knowledge about the Biofield Energy Treatment. The Biofield Energy Treated/Blessed animals were also taken back to experimental room for further proceedings.
- Experimental Procedure
Seven days after acclimatization, animals were randomized and grouped based on the body weight. Dosing for groups G7 and G8 were initiated on day -15 and continued till end of the experiment. However, G1 to G5 and G9 groups were dosed from day 1 till the end of experiment. G6 group was not to be dosed with the test formulation. Body weight and clinical signs were taken daily throughout the experimental period. Feed consumption was measured once in a week. All the animals except G1 group received stress induced procedures such as sound stress, tilted cages and crowd stress, cold and warm water swim stress, food and water deprivation, stress due to change in the light and dark cycle were undergo seven different types of unpredictable stress procedures after scheduled dosing daily at specified interval to the end of the experiment for 8 weeks after the initiation of stress, which vary every week interval i.e., shuffling of stress type. During 8th week of the experimental period, all the animals were individually subjected for blood collection for the experimental purpose.
- Preparation of Sample for Testing Stress Hormone Panel
With the continued stress treatment of 8th week of the experimental period, all the animals were individually subjected for blood collection using retro-orbital route and the blood was collected in the EDTA vial, which was used for the collection of plasma in all the animals of different experimental groups. The plasma from all the groups was stored at -20°C for further estimation. Similarly, CSF was also collected for the estimation of stress hormone panel. Alternatively, aliquot all the samples and store samples at -20°C or -80°C. Avoid repeated freeze-thaw cycles, which may alter the level of stress hormones during final calculations.
- Estimation of UCS Hormone from Plasma (Corticosterone, Angiotensin -II, Nor-epinephrine, Epinephrine) and CSF (Nor-epinephrine)
The plasma and CSF from all the groups was subjected for the estimation of level of stress hormones such as corticosterone, angiotensin -II, nor-epinephrine, epinephrine from plasma and nor-epinephrine from CSF also. The entire stress biomarker hormonal panel was estimated using ELISA method as per manufacturer’s recommended standard procedure. This was a quantitative method and the principle was based on the binding of antigen and antibody in sandwich manner assay.
- Statistical Analysis
The data were represented as mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison One-way analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 was considered as statistically significant.
RESULTS AND DISCUSSION
- Effect of the Test Formulation on Plasma Corticosterone
The role of plasma corticosterone was well established in stress management. Chronic stress increases the plasma corticosterone level, which can be due to various stress-related pathologies such as suppression of reproduction and the immune system, metabolic dysregulation and cognitive impairment [25-27]. UCS model significantly increased the level of corticosterone, and the test formulation significantly maintains the level of hormone. The effect of test formulation on the level of plasma corticosterone was determined and the data are presented in Figure 1. Corticosterone level in unpredictable chronic stress (UCS) G2 group was found to be 176.09 ± 13.1 ng/mL, which was significantly (p≤0.001) increased by 294.4% as compared with the control (G1, 44.46 ± 13.2 ng/mL). Imipramine treatment (G3) significantly (p≤0.01) decreased the corticosterone level (94.88 ± 22.1 ng/mL) by 46.1% as compared to the G2. Untreated test formulation to the untreated rats (G4) showed corticosterone level as 237.64 ± 64.7 ng/mL. Biofield Energy Treated Test formulation to the untreated rats (G5) showed decreased level (119.35 ± 22.5 ng/mL) by 32.2% and 49.8% as compared to the G2 and G4 groups, respectively. Biofield Energy Treatment per se to the rats (G6) significantly decreased the corticosterone level (58.85 ± 7.0 ng/mL) by 66.6% (p≤0.01) and 75.2% (p≤0.05) as compared to the G2 and G4 groups, respectively. 15 days pre-treatment of Biofield Energy Treated Test formulation (G7) showed significant decreased level (138.23 ± 22.6 ng/mL) by 21.5% and 41.8% as compared to the G2 and G4 groups, respectively. 15 days pre-treatment of Biofield Energy Treated Test formulation to the Biofield treated rats (G8) group showed significant decreased corticosterone level (123.79 ± 21.2 ng/mL) by 29.7% and 47.9% as compared to the G2 and G4 groups, respectively. Untreated Test formulation to the Biofield Energy Treated per se rats (G9) decreased (169.42 ± 23.9 ng/mL) by 3.8% and 28.7% as compared to the G2 and G4 groups, respectively.

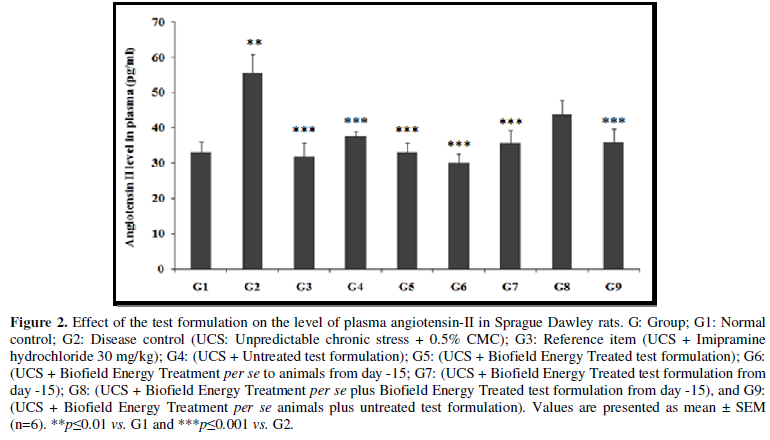
Effect of the Test Formulation on Plasma Angiotensin-II

Effect of the Test Formulation on Plasma Angiotensin-II
One of the important stress hormones is the angiotensin II, which is significantly increased in acute and chronic stress. This can lead to many clinical implications with respect to the kidney and heart physiology-related to inflammation, tissue injury, autoimmunity, oxidative stress and aging [28,29]. The effect of test formulation on the level of plasma angiotensin II was determined and the results are compiled in the Figure 2. Plasma angiotensin II level in UCS group (G2) was 55.67 ± 5.3 pg/mL, which was significantly (p≤0.01) increased by 67.9% in comparison with the normal control (G1) 33.15 ± 3.0 pg/mL. Imipramine treatment (G3) significantly (p≤0.001) decreased the plasma angiotensin II level (31.80 ± 3.9 pg/mL) by 42.9% as compared to the G2. G4 group was reported with significantly (p≤0.001) decreased plasma angiotensin II level (37.57 ± 1.5 pg/mL) by 32.5% as compared to the G2. Similarly, G5 (33.05 ± 1.5 pg/mL) group showed significant (p≤0.001) decreased of percentage of plasma angiotensin II by 40.6% and 12.0% as compared to the G2 and G4 groups, respectively. G6 group showed significantly (p≤0.001) decreased plasma angiotensin II (30.07 ± 2.7 pg/mL) by 46% and 20% as compared to the G2 and G4 groups, respectively. G7 group showed significantly decreased plasma angiotensin II (35.70 ± 3.6 pg/mL) by 35.9% (p≤0.001) and 5% as compared to the G2 and G4 groups, respectively. G8 (43.68 ± 4.1 pg/mL) and G9 (35.91 ± 3.8 pg/mL) group showed significantly decreased plasma angiotensin II by 21.5% and 35.5%, respectively as compared to the G2. G9 group showed decreased plasma angiotensin II by 4.4% as compared to the G4.

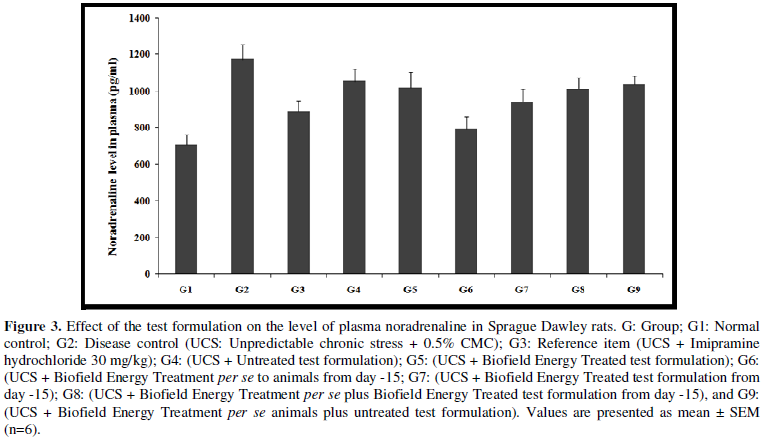
- Effect of the Test Formulation on Plasma Noradrenaline
The role of noradrenaline in stress or psychological conditions was well established and they play a vital role in cardiovascular diseases [30,31]. Many disorders are directly linked with clinical pathologies such as chronic active hepatitis, asthmatics, Crohn's disease, ulcerative colitis, trigeminal neuralgia, chronic relapsing hepatitis, multiple sclerosis, systemic lupus erythematous (SLE), and rheumatoid arthritis (RA). The effect of test formulation on the level of plasma noradrenaline was determined and the results are compiled in the Figure 3. Plasma noradrenaline level in the UCS group (G2) was 239.57 ± 22.3 pg/mL, which was increased by 29.1% as compared with the normal control (G1, 185.64 ± 36.6 pg/mL). Imipramine treatment (G3) decreased the plasma noradrenaline level (134.42 ± 26.0 pg/mL) by 43.9% as compared to the G2. Untreated test formulation to untreated rats (G4) showed value as 305.69 ± 41.8 pg/mL. Besides, G6, G7, and G8 groups showed decreased the plasma noradrenaline level by 13.6%, 20.2%, and 28.4%, respectively as compared to the G4 group.
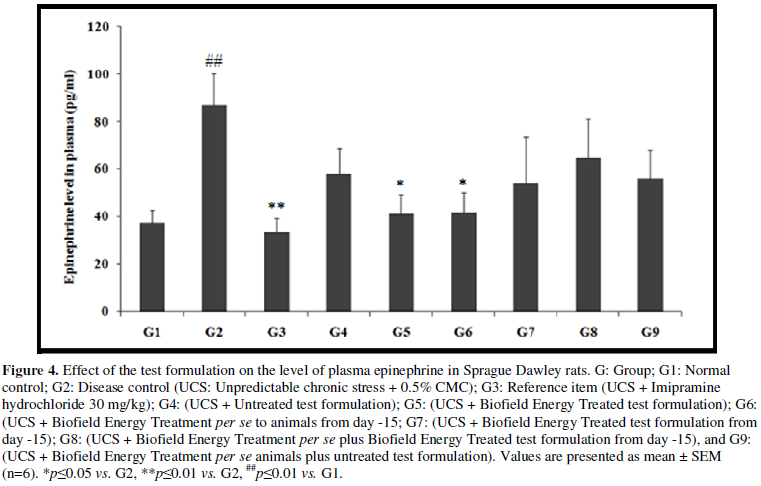
- Effect of the Test Formulation on Plasma Epinephrine
The effects of mental and physical stress have significant impact on plasma epinephrine. It has been reported that increases in circulating epinephrine or adrenaline have been linked with various level of physical sensations (symptoms) which are associated with acute and chronic stress [32]. The effect of the test formulation on the level of plasma epinephrine was determined and the results are compiled in the Figure 4. Plasma epinephrine level in the UCS group (G2) was 86.94 ± 13.17 pg/mL, which was significantly (p≤0.01) increased by 132.2% in comparison with the normal control (G1, 37.44 ± 5.37 pg/mL) group. Imipramine treatment (G3) significantly (p≤0.01) decreased the plasma epinephrine level (33.6 5 ± 5.50 pg/mL) by 61.3% as compared to the G2. G4 group was reported with decreased plasma epinephrine level (58.14 ± 10.57pg/mL) by 33.1% as compared to the G2. Similarly, G5 (41.32 ± 7.81 pg/mL) group showed significant (p≤0.05) decreased percentage of plasma epinephrine by 51.9% and 28.1% as compared to the G2 and G4 groups, respectively. G6 group showed significantly (p≤0.05) decreased plasma epinephrine (41.82 ± 8.12 pg/mL) by 51.9% and 28.1% as compared to the G2 and G4 groups, respectively. G7 group showed decreased plasma epinephrine (54.20 ± 19.23 pg/mL) by 37.7% and 6.8% as compared to the G2 and G4 groups, respectively. G8 (64.71 ± 16.33 pg/mL) and G9 (56.21 ± 11.96 pg/mL) groups showed decreased plasma epinephrine by 25.6% and 35.4%, respectively as compared to the G2 group.


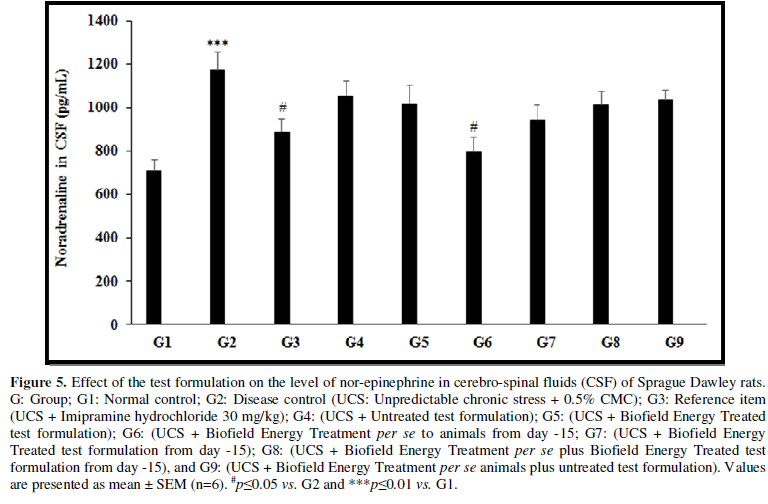
- Effect of the Test Formulation on CSF Norepinephrine
Stress disorder along with blood pressure and cerebrospinal fluid also contribute to affect the level of norepinephrine in CSF [33]. The effect of the test formulation on the level of CSF norepinephrine was determined and the results are compiled in the Figure 5. CSF norepinephrine level in the UCS group (G2) was 1172.27 ± 82.73 pg/mL, which was significantly (p≤0.001) increased by 65.5% in comparison with the normal control (G1, 708.19 ± 52.98 pg/mL). Imipramine treatment (G3) significantly (p≤0.05) decreased the CSF nor-epinephrine level (888.08 ± 58.76 pg/mL) by 24.2% as compared to the G2. G4 group was reported with decreased CSF norepinephrine level (1052.01 ± 71.23pg/mL) by 10.3% as compared to the G2. Similarly, G5 (1017.57 ± 84.78 pg/mL) group showed decreased percentage of CSF norepinephrine by 13.27% and 3.3% as compared to the G2 and G4 groups, respectively. G6 group showed significantly (p≤0.05) decreased CSF norepinephrine (795.34 ± 65.66 pg/mL) by 32.2% and 24.4% as compared to the G2 and G4 groups, respectively. G7 group showed decreased CSF norepinephrine (941.60 ± 69.78 pg/mL) by 19.7% and 10.5% as compared to the G2 and G4 groups, respectively. G8 (1011.93 ± 61.13 pg/mL) group showed decreased CSF nor-epinephrine by 13.7% and 3.8% as compared to the G2 and G4, respectively. G9 (1037.23 ± 43.81 pg/mL) group showed decreased CSF nor-epinephrine by 11.5% as compared to the G2.

In this research plan, four groups were considered as preventive maintenance groups. These groups were G6 (Biofield Energy Treatment per se to animals at -15 days), G7 (Biofield Energy Treated test formulation from day -15), G8 (Biofield Energy Treatment per se to animals along with Biofield Treated test formulation from day -15), and G9 (Biofield treatment per se at -15 days to animals with untreated test formulation). The results showed a significant slowdown of disease progression and all other disease-related symptoms/complications and also reduced the chances of disease susceptibility in these groups. Specifically, group G6 (preventive Biofield Energy Treatment group per se at -15 days) showed the best results as a preventive treatment group compared to the other groups. Based on the overall data, it suggests that the Biofield Energy Healing Therapy was found to be most effective and beneficial to prevent and protect from the occurrence of any type of disease in the rat model. The data indicated that this therapy could act as a preventive maintenance therapy to prevent the occurrence of disease, slowdown the disease progression when disease-related complications are present which will ultimately improve the overall health and quality of life.
CONCLUSIONS
The present study demonstrated the effect of Biofield Energy Treated test formulation and Biofield Energy per se for the estimation of stress hormone that showed significant improved maintenance of the hormonal level, which have significant clinical role in stress-related disorders. Plasma corticosterone level was significantly decreased by 49.8%, 75.2%, 41.8%, 47.9% and 28.7% in the G5, G6, G7, G8, and G9 groups respectively, as compared with the untreated test formulation (G4) group. The data of plasma angiotensin-II showed significant reduced plasma level by 40.6% (p≤0.001), 46% (p≤0.001), 35.9% (p≤0.001), 21.5%, and 35.5% (p≤0.001) in the G5, G6, G7, and G9 groups, respectively as compared with the G4. In addition, plasma noradrenaline level was reduced by 13.6%, 20.2%, and 28.4% in the G6, G7, and G8 groups, respectively compared to the G4. Plasma epinephrine level was significantly reduced by 51.9% (p≤0.05), 51.9% (p≤0.05), 37.7%, 25.6%, and 35.4% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G2 group. Norepinephrine level in CSF was significantly decreased by 13.27%, 32.2% (p≤0.05), 19.7%, 13.7%, and 11.5% in the G5, G6, G7, G8, and G9 groups, respectively compared to the G2 group. Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed the best results with respect to different beneficial efficacy and biomarker parameters in the preventive maintenance group, G6, as compared to the other preventive maintenance groups (G7, G8, and G9) in the rat model study. The Biofield Energy Healing Treatment also helped to slowdown the disease progression and disease-related complications impacting the overall animals’ health. These data suggested that Biofield Energy Treatment per se and Biofield Energy Treated Test formulation in combination would be the best treatment strategy to prevent and protect from the occurrence of any type of diseases. Therefore, the Biofield Energy Healing Treatment (the Trivedi Effect®) per se might be effective in healthy humans, when used as a preventive maintenance therapy to sustain good health, to boost overall health, promote healthy aging and increase quality of life. In the presence of disease, the Biofield Energy therapy might reduce the severity of any acute/chronic disease (such as auto-immune-related and inflammatory disorders) and / or slow the disease progression. This test formulation can be used against systemic lupus erythematosus, fibromyalgia, Addison disease, multiple sclerosis, myasthenia gravis, pernicious anemia, aplastic anemia, psoriasis, rheumatoid arthritis, Crohn’s disease, vitiligo, chronic fatigue syndrome and alopecia Areata, as well as inflammatory disorders such as ulcerative colitis, atherosclerosis, dermatitis, hepatitis, and diverticulitis. However, Biofield Energy Healing Treated test formulation and Biofield Energy Healing Treatment per se can also be used in the prevention of brain disorders such as Alzheimer’s disease, dementias, brain cancer, epilepsy and other seizure disorders, mental disorders, Parkinson’s and other movement disorders, stroke and transient ischemic attack and in the improvement of overall health and quality of life.
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Ruan JW, et al. (2005) Prevalence, correlates, co-morbidity, and comparative disability of DSM-IV generalized anxiety disorder in the USA: Results from the National epidemiologic survey on alcohol and related conditions. Psychol Med 35: 1747-1759.
- Kendler KS, Karkowski LM, Prescott CA (1998) Stressful life events and major depression: Risk period, long-term contextual threat, and diagnostic specificity. J Nerv Ment Dis 186: 661-669.
- Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE (2007) The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn 65(3): 209-237.
- McEwen BS (2006) Protective and damaging effects of stress mediators: Central role of the brain. Dialogues Clin Neurosci 8(4): 367-381.
- Kalivas PW, Duffy P (1995) Selective activation of dopamine transmission in the shell of the nucleus accumbens by stress. Brain Res 675(1): 325-328.
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, et al. (2009) Chronic stress causes front striatal reorganization and affects decision-making. Science 325(5940): 621-625.
- Erb S, Stewart J (1999) A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci 19: RC35.
- Chappell P, Smith M, Kilts C, Bissette G, Ritchie J, etal. (1986) Alterations in corticotropin-releasing factor-like immunoreactivity in discrete rat brain regions after acute and chronic stress. J Neurosci 6(10): 2908-2914.
- Yang G, Wan Y, Zhu Y (1996) Angiotensin II--an important stress hormone. Biol Signal 5(1): 1-8.
- Yang G, Xi ZX, Wan Y, Wang H, Bi G (1993) Changes in circulating and tissue angiotensin II during acute and chronic stress. Biol Signal 2(3): 166-172.
- Sadowski RN, Jackson GR, Wieczorek L, Gold PE (2009) Effects of stress, corticosterone, and epinephrine administration on learning in place and response tasks. Behav Brain Res 205(1): 19-25.
- Seki K, Yoshida S, Jaiswal MK (2018) Molecular mechanism of noradrenaline during the stress-induced major depressive disorder. Neural Regen Res 13(7): 1159-1169.
- Rubik B (2002) The biofield hypothesis: Its biophysical basis and role in medicine. J Altern Complement Med 8: 703-717.
- Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R, et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. Glob Adv Health Med 4: 58-66.
- Evans M, Shaw A, Thompson EA (2007) Decisions to use complementary and alternative medicine (CAM) by male cancer patients: Information-seeking roles and types of evidence used. BMC Complement Altern Med 7: 25.
- Gu S, Pei J (2017) Innovating Chinese herbal medicine: From traditional health practice to scientific drug discovery. Front Pharmacol 8: 381.
- O'Mathúna D (2001) The best of both approaches. The role of science in complementary and alternative medicine. EMBO Rep 2(12): 1054-1057.
- Kumar MT, Alice B, Dahryn T, Snehasis J (2021) Effect of consciousness energy healing treatment on the metal profile and properties of tellurium. Eng Technol Open Acc 3(5): 555623.
- Mahendra KT, Alice B, Dahryn T, Snehasis J (2021) Consciousness energy healing treatment impacted the isotopic abundance ratio of 6-Mercaptopurine (6-MP). Nov Appro Drug Des Dev 5(5): 555673.
- Trivedi MK, Branton A, Trivedi D, Nayak G, Mondal SC, et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indica). J Food Nutr Sci 3: 245-250.
- Trivedi MK, Jana S (2021) Anti-aging activity of biofield energy treated novel proprietary test formulation by assessment of vital biomarkers in cerebrospinal fluid (CSF) in Sprague Dawley rats. On J Neurol Brain Disord 5(2): 2021.
- Kumar MT, Snehasis J (2021) Evaluation of biofield energy healing treatment based proprietary test formulation on gut health potential in colon cancer cell line (HT-29). J Pharmacol Clin Res 8(4): 555743.
- Trivedi MK, Branton A, Trivedi D, Jana S (2021) Isotopic abundance ratio analysis of consciousness energy healing treated folic acid. Food Nutr Current Res 4(2): 290-295.
- Trivedi MK, Branton A, Trivedi D, Jana S (2020) The consciousness energy healing treatment and its impact on the isotopic abundance ratio analysis of flutamide. Drug Des Int Prop Int J 3(5): 2020.
- Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, et al. (2003) Chronic stress and obesity: A new view of ‘comfort food’. Proc Natl Acad Sci USA 100: 11696-11701.
- Martin LB (2009) Stress and immunity in wild vertebrates: Timing is everything. Gen Comp Endocrinol 163: 70-76.
- de Kloet ER, Joëls M, Holsboer F (2005) Stress and the brain: From adaptation to disease. Nat Rev Neurosci 6: 463-475.
- Benigni A, Cassis P, Remuzzi G (2010) Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol Med 2(7): 247-257.
- Arregui A, Perry EK, Rossor M, Tomlinson BE (1982) Angiotensin converting enzyme in Alzheimer's disease increased activity in caudate nucleus and cortical areas. J Neuro Chem 38: 1490-1492.
- Hollenberg NK, Williams GH, Adams DF (1981) Essential HT: Abnormal renal vascular and endocrine responses to a mild psychological stimulus. Hypertension 3: 11-17.
- Folkow B, Di Bona GF, Hjelmdahl P, Toren PH, Wallin BG (1983) Measurements of plasma norepinephrine concentrations in human primary hypertension. Hypertension 5: 399-403.
- Paran E, Neumann L, Cristal N (1992) Effects of mental and physical stress on plasma catecholamine levels before and after β-adrenoceptor blocker treatment Eur J Clin Pharmacol 43: 11.
- Strawn JR, Ekhator NN, Horn PS, Baker DG, Geracioti TD Jr (2004) Blood pressure and cerebrospinal fluid norepinephrine in combat-related posttraumatic stress disorder. Psychosom Med 66(5): 757-759.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Ageing and Restorative Medicine (ISSN:2637-7403)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)







