INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic represents a global challenge all over the world. The pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is complex, and research for specific effective therapies is ongoing. In this setting, the use of passive immunization therapy may represent a valid alternative among the therapeutic armamentarium against COVID-19. Products that have been used to confer passive immunity include convalescent plasma, monoclonal antibodies, immune and hyperimmune globulin. COVID- 19 convalescent plasma (CCP) is the product collected from individuals who recovered from this infection, which contains high titer of specific virus neutralizing antibodies. Convalescent plasma (CP) can be infused in individuals with definite clinical diseases to reduce symptoms and mortality [1,2].
Proven experience with CP in fighting infectious diseases dates back to 1890s, while more recently CP has been used as an empirical treatment for Ebola virus infection during outbreaks in 2014 as well as for other viral infections, including SARS-Co-V, H5N1 avian influenza, and H1N1 influenza, showing efficacy in terms of shorter hospital stay and lower mortality [3]. Plausible explanation for CP therapeutic efficacy relies in the putative activity of virus-specific neutralizing antibodies, which might result in direct clearance of the virus [4]. Viral inactivation may occur through a direct antibody-antigen interaction and/or through the activation of different immunological pathways such as complement activation, antibody-dependent cellular cytotoxicity, or phagocytosis. Finally, anti-inflammatory effects of CP include network of autoantibodies and control of an overactive immune system [5].
Passive immunotherapy using hyperimmune CP from COVID-19 recovered subjects has been largely explored and recently reviewed [6]. As reported, it is difficult to make definitive conclusions about the clinical value (efficacy) of this therapeutic approach, but all data point to an undoubtedly safe profile of CCP transfusions. According to recently published data, favorable clinical outcomes can be achieved when CCP is given earlier in the course of the disease [7], in non-critically ill patients [8], and with a high content of anti- SARS-CoV-2 antibody [9]. However, most of this information derives from studies conducted in adult individuals. Few data on COVID-19 in pediatric population exist [10], no guidelines are available and less is known about the efficacy and safety of CCP in children.
In children, severe COVID-19 is less common than in adults, due to several factors [11]. First, children have a lower expression of angiotensin I converting-enzyme 2 (ACE2), the receptor for SARS-CoV-2 entry into the host cells, and a high number of B and T regulatory lymphocytes that reduces the likelihood of developing severe cytokine inflammatory reactions (i.e., the so-called “cytokine storm”).
This scenario is completely different for children with immune dysfunction, as it occurs in acute leukemia at onset or during its treatment. The reduced immune response exposes acute leukemia patients to a greater risk of infections, and they are more likely to have a poor prognosis when infected with SARS-CoV-2 [12, 13]. In addition, vaccination maybe significantly less effective [14]. In these patients, transfusion of CCP may represent a possible option for pathogen-specific immunotherapy [15].
We report a single-center experience with 6 pediatric cases of children with acute lymphoblastic leukemia (ALL) and SARS-CoV2 infection treated with 1 to max 3 units of CCP, given on a every other day basis. All patients also received an empiric treatment for COVID-19 that included antibiotics and corticosteroid, as per institutional protocol.
METHODS
Study subjects
Children with ALL admitted to the Pediatric Oncologic Unit at Hospital Papa Giovanni XXIII in Bergamo, Italy, with simultaneous confirmed laboratory diagnosis of SARS-CoV-2 infection were considered for CCP treatment. To deliver usual anticancer care while minimizing transmission of COVID-19, nasopharyngeal (NP) swab sampling for SARS-CoV-2 of each child admitted to oncologic division was performed before any elective diagnostic/therapeutic procedures. Testing for SARS-CoV-2 was performed by reverse transcriptase-polymerase chain reaction (RT-PCR) for SARS-CoV-2 nucleic acid. Recovery from COVID-19, was defined as an afebrile status for at least 3 days, alleviation of respiratory symptoms, negative for SARS-CoV-2 nucleic acid for consecutive two RT-PCR tests, and at least 3 weeks following disease onset. Chemotherapy was administered according the “International collaborative treatment protocol for children and adolescents with acute lymphoblastic leukemia “AIEOP-BFM ALL 2017” with the Protocol IA induction phase (including vincristine, daunomycin, pegylated-asparaginase, prednisone). Starting from May 2020 to January 2021, 6 children presented with ALL and simultaneous laboratory confirmed COVID-19 diagnosis by RT-PCR on swab sampling. They received CCP according to the schedule reported below. The ethical conduct of study was performed according to the last revision of the Helsinki Declaration and approved by the institutional review board.
CCP preparation and administration
Convalescent subjects who had had a laboratory-confirmed COVID-19 diagnosis and were fully recovered and negative for SARS-CoV2 NP swab from more than 14 days, voluntarily responded to our plasma donation campaign (launched by the Unit of the Immunohematology and Transfusion Medicine of the Hospital Papa Giovanni XXIII). CCP-donor specific criteria were the following: age between 18 and 65 years, being eligible for blood donation, having been recovered from COVID-19, as assessed by two negative RT-PCR tests on NP swabs before hospital discharge, and having an adequate level of neutralizing anti-SARS-CoV-2 antibodies. Individuals with previous blood transfusions, and women with previous pregnancy or miscarriage history were excluded from donation. CCP collection was performed by standard plasmapheresis procedure, after providing signed informed consent. Each plasmapheresis unit (volume 600 mL) was divided in 3 subunits of 200 mL each and exposed to viral inactivation, frozen, and stored at -30°C. CCP apheresis, pathogen inactivation, freezing, and storage were carried out at the Unit of the Immunohematology and Transfusion Medicine of the Hospital Papa Giovanni XXIII in Bergamo, according to the guidelines of the Italian National Blood Centre (Centro Nazionale Sangue, CNS) [16]. Each CCP unit underwent molecular and serology biological qualification tests (HBV, HIV, HCV serology and molecular, Syphilis serology), in addition were also included HEV, HAV and Parvovirus B19- DNA. Anti-SARS-CoV-2 nucleocapsid antibodies (anti-N) were determined with the SARS-CoV-2 IgG Kit from Abbott on an Architect system and results were expressed as index (positive > 1.4), while anti-SARS-CoV-2 Spike protein (anti-S) antibodies were determined with the LIAISON® SARS-CoV-2 S1/S2 IgG kit (DiaSorin, Italy) and results expressed as AU/ml (positive > 15 AU/ml).
CCP infusion protocol consists in the administration of a maximum 3 AB0-compatible CCP units, every other day [17]. Adverse events associated with infusion were assessed by the treating clinician. During transfusion, patients were under continuous supervision, with vital signs checked periodically. Antibody concentration was measured in patient’ serum samples collected at the following time points: T1 (= baseline, just before the first CCP infusion), T2 (= after 3 days from the first CP, before the second CCP infusion), T3 (= after 5 days from the first CCP, before the third CCP infusion) and T4 (= after 7 days from the first infusion). In one case (i.e., case # 6) additional blood samples were collected in sodium citrate tubes for the measurements of selected coagulation parameters sensitive to inflammation (i.e., fibrinogen and factor VIII), hypercoagulability (i.e., D- dimer) and endothelial activation (i.e., von Willebrand factor, VWF).
RESULTS
Tables 1 & 2 summarize the clinical and laboratory characteristics of the 6 hospitalized ALL pediatric patients, 3 males and 3 females, with a median age of 4.5 years (range: 2-8 years). Three of them had an asymptomatic form of SARS-CoV2 infection, 2 were mildly ill with typical pneumonia sign on chest imaging, while the last case had a COVID-19 severe respiratory failure. The clinical cases are described below in chronological order.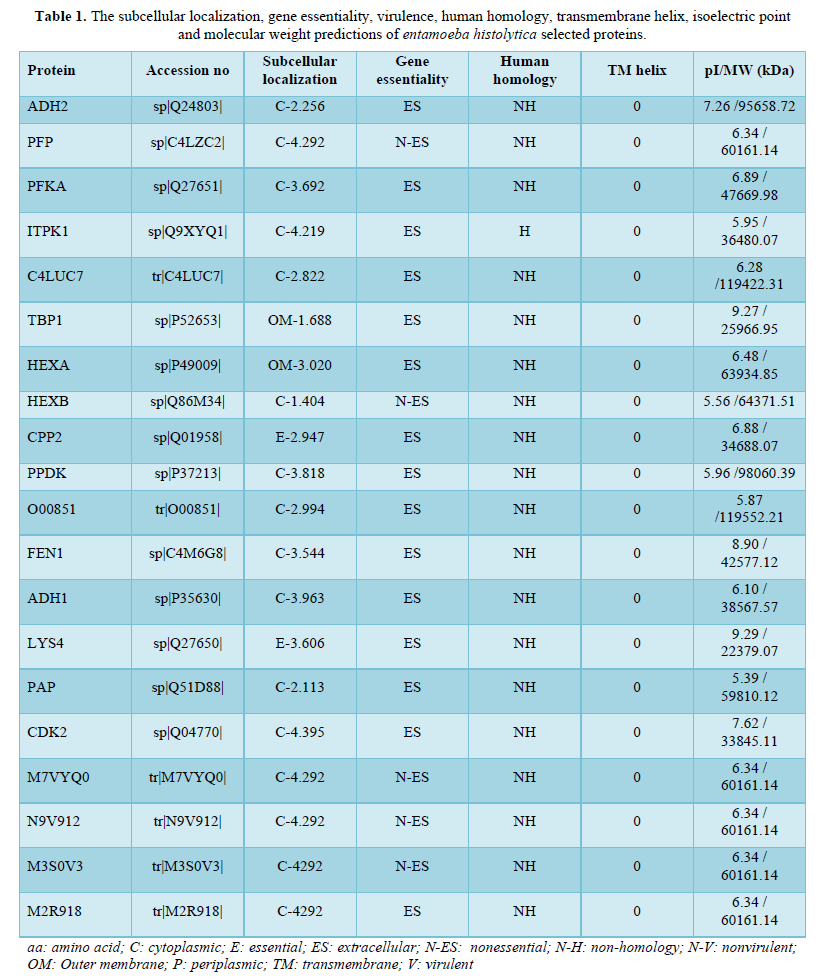
Case #1
A 7-year-old female was hospitalized on May 23, 2020, after a longer than one-month history of fever, fatigue, dry cough, and enlargement of later cervical lymph nodes associated with pain. Her blood tests showed neutrophilia with 35% of blast cells, anemia and thrombocytopenia, and increased levels of lactate dehydrogenase (LDH), C reactive protein, and D-dimer. An ALL, developed from the precursor B-cell, EGIL-B II (common) (CD10+, CD19+, CD58+, cyCD79a+, CD45+, CD34+, CD22+, HLA-DR +, CD38+, CD9 +), without central nervous system (CNS) localization, was diagnosed. No BCR/ABL1 p190, BCR/ABL1 p 210, MLL-AF4, TEL-AML1, E2A-PBX1, E2A-HLF translocation was observed.
Chemotherapy was started according the AIEOP-BFM ALL 2017 with the Protocol IA induction (with methylprednisolone). On May 27, chest computed tomography (CT) scan showed remarkable extension of lung infiltrates and NP swab and serological test were positive for SARS-CoV2. On the same day, parents were negative at SARS-CoV-2 NP. Given the very critical condition of the child due to leukemia and pneumonia, it was decided for CCP administration. The patient received 3 units of CCP, with no adverse events. On day 4, after the second infusion, 2 subsequent NP swabs were performed both of which tested negative. A new chest CT scan, performed 9 days after the first CP infusion, documented almost complete resolution reduction of lung infiltrates and a significant reduction of the lymph nodes.
The chemotherapeutic program was regularly continued according to the Induction phase of the protocol. At the revaluation on day 8 a prednisone good response was observed, on day 15 the Flow Cytometric Minimal Residual Disease (FCM-MRD) resulted negative for lymphoid blasts (i.e., 0.6%), and a complete remission was achieved one month later the ALL diagnosis. Child clinical condition improved and, on June 16, she was discharged to continue therapy at home.
Case #2
A 2-year-old female was admitted to hospital on Jun 3, 2020, for fever, walking difficulty, inguinal lymph-adenomegaly and splenomegaly, with petechiae on arms and limbs. The blood examination
Revealed hyperleukocytosis with 94% blasts, neutropenia, anemia, thrombocytopenia, and increased levels of lactic dehydrogenases, C reactive protein and D-dimer. An ALL, developed from the precursor B-cell, EGIL-B II (common) (CD10+, CD19+, CD58+, cyCD79a+, CD45+, CD34+, CD22+, HLA-DR +, CD38+, CD9 +) with CNS localization (blast 21%), was diagnosed. NP swab performed at hospital admission reacted positive SARS-CoV2. Her father had SARS-CoV-2 confirmed infection 3 months before, while at the admission of the child, parents were both negative at SARS-CoV2 NP. The chest X-ray showed a peribronchial thickening - densification.
The prophase therapy with escalating doses of sole steroid (methylprednisolone at the initial dosage of 40 increased up to 60 mg/m2/day) was promptly started. Nevertheless, one day later, white blood cell count increased to 522 x109/L. meantime, the molecular studies revealed the presence of BCR- ABL1 p190 translocation, confirmed by the cytogenetic analysis. Thus, according to the international EsPhALL2017 protocol, the tyrosine kinase inhibitor Imatinib (340 mg/ m2/day) in association to steroid was planned. Given urgent need to start chemotherapy, CCP infusion, 3 units, was performed starting from June 8. No adverse event occurred. The subsequent 2 NP swabs, tested at day 2 and 3 after the first CCP infusion, were both negative. The chemotherapy continued with the expected intensity, and after the first month of treatment, the child reached the complete remission of the disease, in association with the resolution of the chest imaging of the lung lesions. On July 10, the child was discharged.
Case #3
A 5-year-old male with recent diagnosis (June 12, 2020) of common ALL, without CNS localization. The molecular analysis showed the absence of the BCR/ABL1, TEL/AML1, E2A/PBX1, MLL/AF4, MLL/AF9, MLL/ENL, E2A, HLF rearrangements. He started the Induction A chemotherapy according AIEOP-BFM ALL 2017. Complete remission was obtained one month after the ALL diagnosis at the end of Induction A protocol. The PCR-based MRD detection by clone-specific junctional regions, classified the patient as early high risk. On the bases of this result the child had to receive a more intensive chemotherapy program with an extended consolidation.
On August 3, 2020, the patient was admitted to day-hospital to start consolidation. After routine check, SARS-CoV2 NP resulted positive. The child had no symptoms attributable to the COVID-19 and was negative at the chest X-ray imaging. Given his chemotherapy-associated immunosuppression state, it was decided to start CCP infusion. After the first infusion, a mild rash appeared which led to discontinuation of treatment. The rash receded with antihistamines and steroids; and no other CCP infusions were given. However, 2 NP swabs performed after the first
Infusion was both negative for SARS-CoV2. On August 8, the child was discharged in good clinical condition.
Case #4
A 7-year-old male with recent diagnosis (April 24, 2020) of common B-ALL, with CNS localization, receiving chemotherapy according AIEOP-BFM ALL 2017 protocol was admitted on September 15 to the hospital to start the HD-MTX third cycle of extended consolidation protocol. At admission, SARS-CoV2 NP swab and the serological test resulted positive. The mother was also found positive at SARS-CoV2 NP. The chest X-ray of the child showed parabronchial infiltration. The child then received CCP infusion starting from September 16. After the second CCP infusion, the two NP swabs performed resulted negative and on September 20 it was possible to start the third chemotherapy cycle. No adverse events occurred, the third chemotherapy cycle was administered, and the child was discharged in good clinical condition.
Case #5
A 4-year-old female with diagnosis of B-ALL on July 07, 2020, without SNC localization, receiving chemotherapy according AIEOP-BFM ALL 2017 protocol. At the beginning of January 2021, in the chemotherapy maintenance phase, during routine screening, she tested positive for the NP swab. The chest radiography was normal, and no other symptoms was reported associated to COVID-19. Because her chemotherapy-associated immunosuppression state, it was decided to start a prophylactic therapy with antibiotics and then she was enrolled in the CP protocol. Child was discharged home, despite the NP swab positivity, as she was in good clinical condition.
Case #6
A 2-year-old male was diagnosed with ALL with CNS localization on January 05, 2021, and chemotherapy according AIEOP-BFM ALL 2017 protocol was started. Few days after chemotherapy start, he developed fever, cough, and dyspnea. Chemotherapy was stopped and the patient was hospitalized. The chest CT scan showed remarkable extension of lung infiltrates compatible with viral pneumonia, NP swab tested positive, antibiotic therapy was started. Because of breathing worsening, the patient was transferred to our hospital, he was admitted to ICU where non-invasive ventilation with CPAP was started. In addition, remdesivir and anticoagulants were administered. CP infusion was started, and after the second infusion, CPAP was stopped and after 2 days, no ventilatory support was needed anymore. No adverse events to CP transfusion were registered. Patient clinical condition improves and after ten days, on 28 of January, the child return to Monza hospital to continue chemotherapy for LLA.
Anti-SARS-CoV2 antibodies
Figure 1 shows the levels of anti-S (left panel) and anti-N (right panel) antibodies measured at baseline, before the first infusion of CCP unit, and after 7 days. Three cases (i.e., 1, 2 and 6) were positive for the presence of both anti-S and anti-N antibodies at baseline, while the remaining 3 cases were negative. After 1 week from the first CCP infusion, both anti-S and anti-N antibody concentrations increased. The values observed in circulation of each patient was the sum of autologous antibodies plus those acquired from the CCP infusion. Indeed, a good correlation between levels of circulating anti-S antibodies and the expected levels based on CCP anti-S content was observed (R2= 0.783; p<0.01).
Biochemical analyses
Results of biochemical parameters testing during observation week do not highlight significant differences in any of the study subjects (Table 2). Differently, an improvement in the proinflammatory profile was observed in 2 patients (i.e., case 1 & 6) and was characterized by a decrease in ferritin and LDH values, although some data are not available. Furthermore, analysis of coagulation profile performed in Case # 5 showed a significant reduction of fibrinogen (443 vs 230 mg/dL), FVIII (240 vs 119 %), D-dimer (261 vs 173 ng/ml), vWF activity (184 vs 125%) and vWF antigen (210 vs 136%) towards normal values after 7 days from the first CP infusion (Figure 2).
DISCUSSION
In this study we report on 6 consecutive cases of children with ALL and concomitant SARS-CoV-2 admitted at single institution. At admission, the common manifestation was fever that is not specific and indistinguishable from the that observed in children presented with infection during pancytopenia secondary to chemotherapy, and one of the common symptoms at the onset of a leukemia. From a biochemical perspective, they had leukopenia with marked lymphopenia, thrombocytopenia, and increased ferritin. Only the regular screening for SARS-CoV-2 performed by our institution was able to identify the infected patients. In all these children, the infusion of CCP, aiming to provide virus-specific neutralizing antibodies, contributed to a prompt improvement and recovery of symptoms. A recent retrospective analysis showed that CCP therapy was associated with a survival benefit in a large cohort of adult patients with hematologic cancers and COVID-19 [18]. To our knowledge, such treatment in COVID-19 has been previously reported in only 2 children with ALL [15]. Among the 6 cases described in our report, it is possible to distinguish two types of conditions, i.e., children in whom the onset of leukemia was concomitant with the diagnosis of SARS-CoV2 infection, and children that were already undergoing chemotherapy at the time of viral infection.
Independently of the condition type, providing a rapid recovery from the viral infection was of crucial importance in our patients, first for starting or continuing as soon as possible the antitumor treatment and, second, for supporting the compromised immune system and avoid virus spreading or disease worsening.
Our data show that the infusion of CP contributed to a complete viral clearance within few days (11 days on average) without the use of any specific antivirals that was followed by a prompt improvement of symptoms. This finding was more evident in the child with severe COVID-19 (Case #6) that started CCP infusion while he was on non-invasive mechanical ventilation; indeed, after the infusion of the third unit of CCP, the child experienced an important improvement in clinical condition and oxygen therapy was stopped.
The rapid viral clearance suggests that CCP antibodies might be responsible for the therapeutic effect in our patients, probably through the neutralization of the virus and bridging the gap from adaptive immunity [5]. Our data are in agreement with other case reports, in which CP therapy has been used to treat pediatric patients with other types of immune deficiencies [12,19,20].
Specifically, 3 hospitalized children with X-linked agammaglobulinemia who experienced prolonged COVID-19 course and minimal improvement on supportive therapies, showed rapid clinical improvements shortly after the infusion of CCP [12]. Another important result was achieved in a 4-years old girl with B-ALL and severe hypoxia due to COVID-19 pneumonia (case # 6), in which CCP infusion caused a remarkable improvement in supplemental oxygen and reduction of viral load without the use of any specific antivirals.
Another beneficial effect of CCP infusion in our cases appeared from the analysis of the blood chemistry data, showing a significant reduction in the serum levels of C reactive protein, ferritin and lactate dehydrogenase in a few days, during the observational week, subjects’ inflammatory state as well as clinical manifestation got, dramatically, better. In one case, the extensive analysis of the coagulation profile during CP infusion showed, in addition, a rapid amelioration of the pre- infusion hypercoagulable state and endothelial cell perturbation, that characterized moderate to severe forms of COVID-19 [21]. The measurement SARS-CoV2 antibodies in blood samples collected before the first infusion of CCP and 7 days later, showed a remarkable increase in anti-N and anti-S antibodies. Interestingly, the observed antibody increase is compatible with the calculation, albeit empirical, of the antibodies expected after each CCP transfusion. In our case series, only one minor adverse event occurred in one of the patients and consisted of an urticaria reaction that did not affect the child's hospital stay.
CONCLUSION
In conclusion, to the best of our knowledge, this relatively large case series showed that CCP could be an effective and well tolerated therapy for child with ALL and concomitant SARS-CoV2 infection. As we recognized, the currently available scientific literature in onco-hematological child is limited to few case reports all suggesting a potential benefit for CCP treatment. This type of passive immunotherapy in children is potentially of interest and warrants planning of clinical trials with large number of patients. For the future, with the advent of targeted monoclonal antibodies, it remains to observe whether their role will surpass that of the plasma-based modalities, especially in this high-risk population.
ACKNOWLEDGEMENTS
The study was partially supported by Fondazione ARTET Bergamo, Italy.








