Review Article
Pathogen Induced Thrombotic Microangiopathy
3874
Views & Citations2874
Likes & Shares
Thrombotic microangiopathy is clinically characterized by intense thrombocytopenia and microangiopathic hemolytic anemia. They are mainly categorized into two major disorders: thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). ADAMTS13 deficiencies, mutations in the ADAMTS13 gene or autoantibodies that inhibit ADAMTS13 activity are the major etiology for TTP. This can be triggered by various factors such as sepsis, autoimmune disorders, malignancies, transplant, pregnancy and drugs. However, there are reports of TTP like syndromes with normal ADAMTS13 activity having elevated procalcitonin levels. These findings demonstrate the importance of rapid assays such as PCT and CRP to identify sepsis in a subset of TMA patients.
Keywords: Hemolytic uremic syndrome, Thrombotic thrombocytopenic purpura, Thrombotic microangiopathy, procalcitonin, Sepsis
Abbreviations: TMA: Thrombotic microangiopathy; HUS: Hemolytic uremic syndrome; TTP: Thrombotic thrombocytopenic purpura; PCT: Procalcitonin; CRP: C - reactive protein; DIC: Disseminated intravascular coagulation; ADAMTS13: a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13
INTRODUCTION
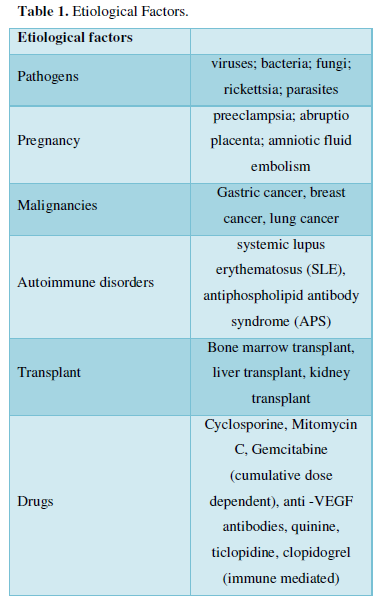
Thrombotic microangiopathy (TMA) includes a heterogeneous group of syndromes characterized by microvascular thrombosis, thrombocytopenia, microangiopathic hemolytic anemia, and organ failure. Common forms of TMA include thrombotic thrombocytopenic purpura (TTP) and the hemolytic uremic syndrome (HUS) [1]. TTP is characterized by microvascular thrombosis associated with markedly decreased ADAMTS13 activity due to mutation of ADAMTS13 gene (hereditary) or due to antibody against ADAMTS13 (acquired) [2]. Acquired thrombotic thrombocytopenic purpura (TTP) is a rare, fatal thrombotic microangiopathy with an estimated incidence of three cases per 1,000,000 adults per year [3]. The disease manifests as thrombocytopenia, hemolytic anemia, and organ failures. Its etiology is unclear, although it has been linked to various conditions such as sepsis, autoimmune disorders, malignancies, transplant pregnancy and drugs. However, thirty to seventy percent of patients diagnosed with TMA do not have severe ADAMTS13 deficiency [4-7]. Hence, the intervention with plasmapheresis which is considered as the lifesaving procedure in the management of TTP could not be considered in this population. Erickson [8], retrospectively analyzed 61 patients with TMA and found that 37% non-ADAMTS13-deficient patient samples were strongly positive for procalcitonin (PCT) [8]. These patient samples also had a >10-fold higher median c-reactive protein (CRP) level than patients with normal PCT. Result of his study demonstrates the importance of rapid assays such as PCT and CRP to identify sepsis in a subset of TMA patients. In contrast to this finding, Krupesh [9] reported a case of normal ADAMTS13 activity TTP with elevated PCT and CRP, though 48 h blood and urine culture was not able to find growth of any organism [9]. TTP is often triggered by infection, and its discrimination from sepsis-associated DIC is not necessarily easy in such cases. In TTP, microthrombi are induced by platelet/von Willebrand factor (VWF) microaggregate formation, and severe platelet depletion is the major characteristic [10]. Common features of TTP consist of thrombocytopenia, MAHA, fluctuating neurological signs, renal impairment, and fever. Since the incidence is far less than that of DIC, TTP is often initially diagnosed as DIC in sepsis patients [11]. Therefore, the need for a prompt diagnostic criterion for differentiating TTP with other TTP like syndrome is very essential (Table 1).

PATHOGEN INDUCED TTP
In an analysis done by Oklahoma registry, they identified 98 patients of TTP with 41 different infectious etiologies. The types of infections were diverse; 31 different bacteria, six different viruses, and four different fungi were identified [12]. TC Nguyen et al has reported that patients with severe sepsis revealed reduced ADAMTS-13 activity and the deficiency correlated with severity of thrombocytopenia and plasma level of interleukin-6 (IL-6) [13]. Pathogens can directly inhibit ADAMTS-13 and allows large complexes of the clotting protein known as von Willebrand factor to circulate in the blood, resulting in platelet clotting and the destruction of red blood cells. However, there exists a subset of TMA patients with normal ADAMTS13 activity. This remains a heterogeneous group of patients in which the appropriate treatment is not well understood. According to the study of Erickson [8] 11 of 30 (37%) non-ADAMTS13-deficient patient samples were strongly positive for Procalcitonin. In addition to indicating the presence of a bacterial infection, their data reveals that elevated PCT in TMA is also associated with a low likelihood of ADAMTS13 deficiency. This finding further supports the observation that TMA with severe deficiency of ADAMTS13 is clinically and pathologically distinct from TMA with normal ADAMTS13 activity. Since TTP is a rare disease, the numbers of available literatures are very limited and it is difficult to decide treatment for these patients especially when they have a normal ADAMTS13 level.
CRP is another biomarker used for inflammation, which is also used along with other procalcitonin in sepsis. Study found out 80% of the TMA patients with elevated CRP which includes both ADAMTS13 deficient and normal group. This further supports the importance of inflammation in the pathogenesis of many forms of TMA.
CONCLUSION
Diagnosis and management of TTP is very difficult if ADAMTS13 is normal. Plasmapheresis is not indicated in those conditions. Preliminary data suggests that procalcitonin and CRP can help in identifying the subset of TTP which is due to septic inflammation. Therefore, it is important to check these biomarkers in advanced large-scale researches on this topic.
- Sadler JE (2008) Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood 112(1): 11-18.
- Chang JC (2018) TTP-like syndrome: Novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb J 16: 20.
- Sravanthi MV, Kumaran SS, Sharma N, Bojanapally P (2020) A Rare Case of Acquired Thrombotic Thrombocytopenic Purpura Triggered by Acute Pancreatitis. Cureus 12(6): e8477.
- Raife T, Atkinson B, Montgomery R, Vesely S, Friedman K (2004) Severe deficiency of VWF-cleaving protease (ADAMTS13) activity defines a distinct population of thrombotic microangiopathy patients. Transfusion 44: 146-150.
- Veyradier A, Obert B, Houllier A, Meyer D, Girma JP (2001) Specific von Willebrand factor-cleaving protease in thrombotic microangiopathies: A study of 111 cases. Blood 98: 1765-1772.
- Vesely SK, George JN, Lämmle B, Studt JD, Alberio L, et al. (2003) ADAMTS13 activity in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome: Relation to presenting features and clinical outcomes in a prospective cohort of 142 patients. Blood 102: 60-68.
- Moore JC, Hayward CPM, Warkentin TE, Kelton JG (2001) Decreased von Willebrand factor protease activity associated with thrombocytopenic disorders. Blood 98: 1842-1846.
- Erickson YO, Samia NI, Bedell B, Friedman KD, Atkinson BS, et al. (2009) Elevated procalcitonin and C-reactive protein as potential biomarkers of sepsis in a subpopulation of thrombotic microangiopathy patients. J Clin Apher 24: 150-154.
- Krupesh VR, Mohanty B, Srinivas BJ, Jadhav S, Basavaraj KH, et al. (2020) Sepsis Mimicking TTP-Case Report and Review of Literature. J Surg Anesth Res 1(2): 1-2.
- Scully M, Hunt BJ, Benjamin S, Liesner R, Rose P, et al. (2012) Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 158: 323-335.
- Wada H, Matsumoto T, Suzuki K, Imai H, Katayama N, et al. (2018) Differences and similarities between disseminated intravascular coagulation and thrombotic microangiopathy. Thromb J 16: 14.
- Booth KK, Terrell DR, Vesely SK, George JN (2011) Systemic infections mimicking thrombotic thrombocytopenic purpura. Am J Hematol 86: 743-751.
- Nguyen TC, Liu A, Liu L, Ball C, Choi H, et al. (2007) Acquired ADAMTS-13 deficiency in pediatric patients with severe sepsis. Haematologica 92: 121-124.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Rheumatology Research (ISSN:2641-6999)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- International Journal of Diabetes (ISSN: 2644-3031)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)


