Research Article
Comparative Evaluation of Platelet Concentrates from Fresh versus Overnight Stored Blood: An Institutional Study
3807
Views & Citations2807
Likes & Shares
The Quality of platelet concentrate (PCs) depends on technique used for preparation, storage bag material, storage period and type of anticoagulant used. This study undertakes the comparative evaluation between platelet concentrates prepared from fresh whole blood versus overnight stored blood of various parameters.
Aims and Objectives: Comparative evaluation between PCs prepared from Fresh WB (6 h) vs. Overnight (24 h) of various morphological and metabolic parameters such as PLT Count, PLT volume, LDH, Bicarbonate etc. respectively during 1st, 3rd and 5th day.
Materials & Methods: Study was done in Our Hospital based blood bank for six months.
Sample size: 50 for each category. Fifty (50) units of WB was taken and stored for greater than 24 h at a temperature of around 22 to 24 and subsequent day processed. Remaining 50 units were immediately processed within 6-8 h. The WB was subjected to centrifugation via “HEAVY” spin. PRP bags were subjected for centrifugation via “LOW” spin. All the samples had undergone mandatory serological testing. All seronegative samples had undergone mandatory quality control checks. Sterility check was done on day 5 of storage.
Statistical Analysis: Comparisons were done on PCs from overnight whole blood versus new (fresh) blood utilizing a two-sample t-test at 95% confidence interval. Comparisons were also evaluated on platelet concentrates from overnight-held Whole blood versus quality control requirement by using one sample t-test. A value of p
Results: Discussed further in the text.
Conclusion: The choice of usage of overnight WB at room temperature must be inclusive of the plasma quality and RBC. Variables show wider standard deviation in our study, so more standardization is needed for PCs processing.
Keywords: Platelet concentrates, Fresh, Overnight
Abbreviations: WB: Whole Blood, PCs: Platelet concentrates, PLT count: Platelet count, MPV: Mean Platelet volume, PDW: Platelet distribution width, TRBC: Total RBC count, TWBC: Total WBC count, RT: Room temperature, LDH: Lactate dehydrogenase, PPP: Platelet poor plasma, PRP: Platelet rich plasma, AABB: American association of Blood banks
INTRODUCTION
Components of Blood are obtained from whole blood (WB) as timely as feasible after collection of blood. The Paramount period of storage before components preparation is around 8 hours at a temperature of around 20°C to 24°C. There is rising intrigue in lengthening the period to remove the necessity for numerous transports runs from the site of collection to the preparation of component. This allows preparation of component units in one shift instead of multitudinous shifts for components sorted from the WB. So, Blood Bank Staffs for preparation of blood components are inherently required solely during regular hours and the burden of work can be uniformly distributed over time. It provides systematic manufacturing of blood components by averting interlude time for WB to be furnished from different blood collection sites. The quality of platelet concentrate (PCs) depends on technique used for preparation, storage bag material, storage period, storage temperature and type of anticoagulant used. This study undertakes the comparative evaluation between platelet concentrates prepared from fresh whole blood versus overnight stored blood of various morphological parameters such as count of platelets (PLT Count), (MPV) Mean Platelet volume and PDW (Platelet distribution width) and comparative evaluation between PCs obtained from WB versus overnight blood for various parameters including Glucose, Lactate, Chloride, Bicarbonate, pH, pO2 and pCO2 [1,2].
Aims and objectives
- Comparative evaluation between PCs prepared from Fresh WB(6hrs) versus Overnight (24hrs) of various morphological parameters such as PLT Count, PLT volume, MPV, PDW, etc. during 1st ,3rd and 5th
- Comparative evaluation between PCs prepared from Fresh WB (6 h) vs. overnight (24 h) of various metabolic parameters such as glucose, lactate, chloride, bicarbonate, along with pH and pO2, pCO2, etc. over a period of 1,3,5 days [3].
MATERIALS AND METHODS
We conducted the prospective study in Blood Bank of Our Hospital for duration of around six months with a sample size of 50 for each category.
Fifty (50) units of WB was taken and stored for greater than 24 h at a temperature of around 22 to 24 and had undergone processing on the subsequent day. Remaining 50 units were immediately processed within 6-8 h. The WB was subjected to centrifugation by using a “HEAVY” spin (3400 rpm for 11 min duration). PRP had to be expressed into the satellite bag which was contemplated for storage of platelets. The PRP bags were subjected for centrifugation at 20 using a “LOW” spin (1000 rpm for 6minutes). All the samples had undergone serological testing under Eci Chemiluminescence (Victors Ortho clinical Diagnostics).
All seronegative samples had undergone mandatory quality control checks to maintain the AABB (American association of Blood Bank) quality standards. Quality evaluation of PCs includes: Platelet count (PLT Count), Total RBC count (TRBC), Total WBC count (TWBC), pH of the platelet concentrates (PCs) was noted on day 1,3,5 along with sterility check on day 5 of storage [2].
pH determination
pH for platelet concentrates evaluated by utilizing pH meter. (Eutech instrument pH tutor). pH meter Electrode was put down in platelet concentrates and agitated the solution. pH reading should be kept stable preliminary to the pH measurement of Platelet concentrates is taken [2].
Platelet count, Total RBC count, Total WBC count
Platelet count, Total RBC count, Total WBC count were evaluated by utilizing (Haematology analyser-Sysmex XN 550). 2 ml of PCs was poured in 4 ml of plain tube and mixing was done appropriately. Cassette cartridge was put firmly into the loading tray of the Haematology analyser. Analyser automatically initiated the cycling of cassette and after completion of the cycle sample results were analyzed and reviewed at the workstation. [2]
Sterility test
This procedure is done to rule out bacterial contamination during fifth day of the Stored PCs by utilizing the automated version of blood culture detection system. 10 ml of PCs was taken by using syringe in sterile conditions, subsequently moved to the culture bottle. First PCs has to be put in anaerobic culture before putting into aerobic culture bottle. The cultured bottle was incubated in automated culture system for 7 days [2].
Statistical analysis
Comparisons were done on PCs from overnight whole blood versus new (fresh) blood utilizing a two-sample t-test at 95% confidence interval. Comparisons were also evaluated on platelet concentrates from overnight held whole blood versus quality control requirement by using one sample t-test. A value of p
RESULTS
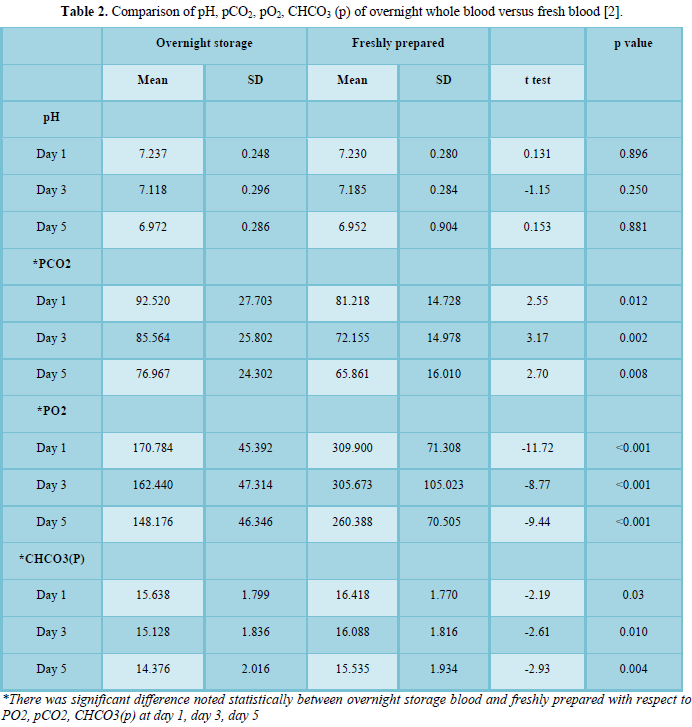
Results of PCs obtained after WB Storage for 24 h at room Temperature (RT) in contrast with the obtained PCs which are fresh was appraised and the inferences are expressed in Table 1.
As p-value was <0.001(p value <0.05), there was sufficient corroboration indicating that the PLT count for PCs from 24 h stored WB was less in comparison with the fresh processed PCs on Day 1,3,5.
PCs obtained from overnight WB had a noteworthy more TRBC on 1st day (p value was less than 0.001), On 3rd day p value was (0.366) and on 5th Day p value was (0.717).
The p value st Day.
PCs prepared from overnight Whole Blood had a notable low PDW on 1st and 5th Day (p value was st and 5th day). PCs prepared from overnight Whole Blood had a notable low MPV on 1st, 3rd and 5th Day as p value was st, 3rd and 5th day) [3].
Our study has observed found that parameters of overnight stored blood (24 h) and fresh WB (within 6-8 h) with respect to PLT Count, PDW, MPV at 1st, 3rd day and 5th day was of statistical significance. Relatable findings were noted with respect to WBC count, RBC count at day 1.
Quality of PC processed from both overnight-held WB and freshly obtained PCs evaluated for other parameters such as pH, pCO2, PO2, and the consequences of the study are shown in Table 2. No substantial statistical difference of significance was noted between the categories of both types in relation to pH as p value was 0.896, 0.250, 0.881 on 1st, 3rd and 5th day respectively.
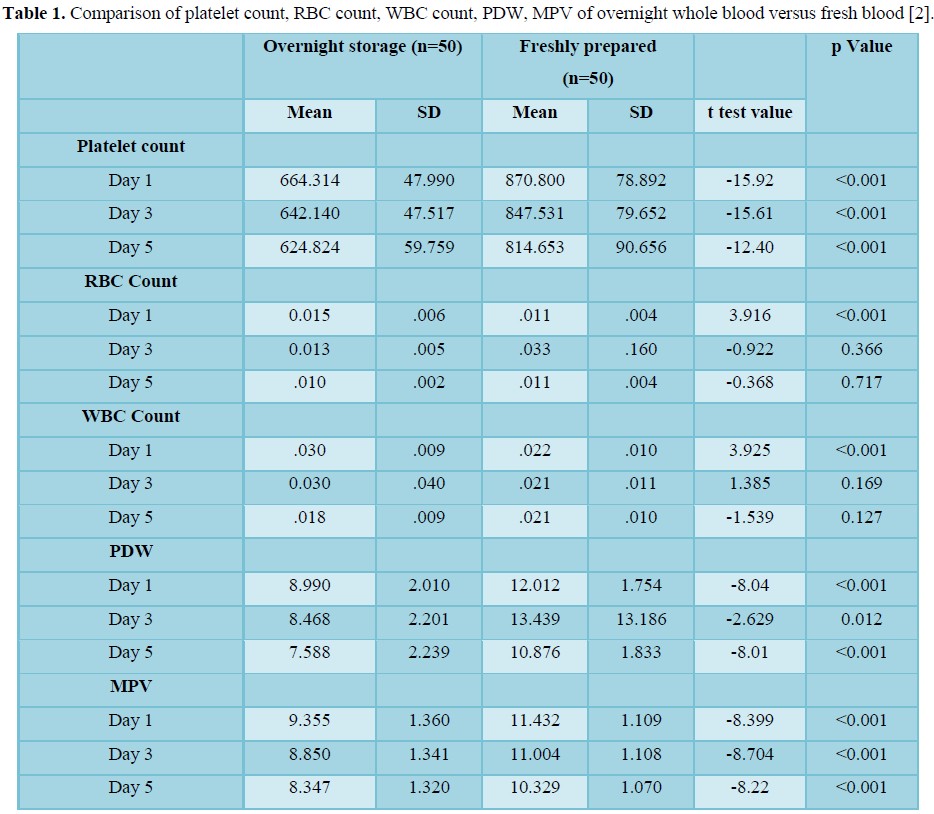
Freshly prepared PCs has notably lower pCO2 on 1st day, 3rd day and 5th day (As the p value was 0.012, 0.002, 0.008 respectively p value <0.05). As p-value was st, 3rd and 5th Day. Also for CHCO3-(P) p-value was < 0.001on all the days Day 1, 3 ,5 (p value <0.05), there was enough corroboration indicating that CHCO3-(P) for PCs from overnight stored WB was less in contrast with the freshly prepared PCs on 1st , 3rd and 5th Day (p values 0.03, 0.010,0.004 respectively on 1st , 3rd and 5th day) [2].

Freshly prepared PCs has notably lower pCO2 on 1st day, 3rd day and 5th day (As the p value was 0.012, 0.002, 0.008 respectively p value <0.05). As p-value was st, 3rd and 5th Day. Also for CHCO3-(P) p-value was < 0.001on all the days Day 1, 3 ,5 (p value <0.05), there was enough corroboration indicating that CHCO3-(P) for PCs from overnight stored WB was less in contrast with the freshly prepared PCs on 1st , 3rd and 5th Day (p values 0.03, 0.010,0.004 respectively on 1st , 3rd and 5th day) [2].
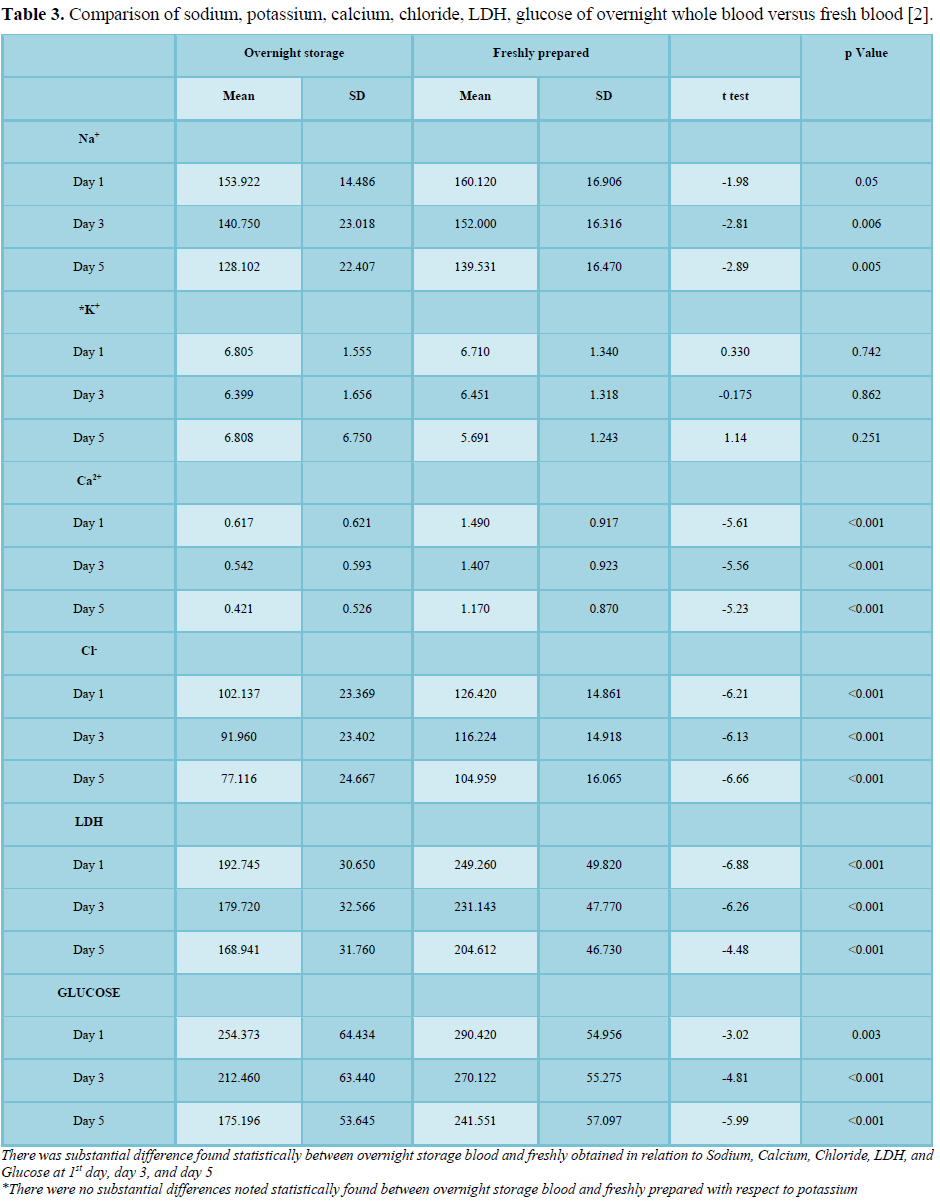
Quality of PC processed from two categories was also appraised for parameters such as Sodium, Potassium, Calcium, chloride, LDH, Glucose and the consequences of the study are shown in Table 3. Freshly prepared PCs as compared to overnight held WB had more sodium, calcium, chloride, LDH and glucose on all the days 1st, 3rd and 5th day. P value for sodium (Na+) was 0.05, 0.006, and 0.005 on 1st, 3rd and 5th day respectively. For calcium, chloride and LDH on all the days p value was rd and 5th day (p value <0.05). There were no substantial differences noted between both the categories in respect to potassium [2].
DISCUSSION
PCs can be obtained from each unit of WB nonetheless from site of collection to the processing centre. Platelets can be processed in a solitary shift therefore reducing the utilizing costs and thereby number of shipments of whole blood from Site of collection can be remarkably reduced [4].

This study emphasizes on the estimation of the quality of PCs produced from overnight WB at room temperature on Day 1st, 3rd and 5th and to see whether the quality of the platelets is influenced by the hold conditions. Three appraisement days (continuous) of PCs along with interval of two days allows monitoring the impact of quality changes which was mainly determined in our study. PLT Count on 1st day for PCs from WB stored for 24 h had noteworthy less PLT count than the freshly obtained PCs. The explanation for decreased platelet yield is the platelet to platelet aggregation is affected there is disaggregation formed during centrifugation and preparation of PCs. [5]
In storage conditions, through 3rd day and 5th day, platelets count for PCs obtained from WB stored for 24 h apparently had similar sequel with freshly obtained PCs. In few other studies platelets obtained from overnight-held WB had more platelet counts showed a 33% greater concentration of platelets [6].

In other studies, active cooling devices were utilized for cooling of the blood to 20°C to 24°C after collection. In our study, we had not utilized active cooling devices can be the reason of having lower yield of platelet count. Few studies show the temperature control by active cooling devices may influence the count of platelets. Although platelet count in PCs unit processed from stored WB for 24 h period can be used for patients having thrombocytopenia. TRBC in this study was remarkably more on Day 1 from stored WB as compared to freshly obtained PCs. The PCs were being obtained from WB via soft-spin for separation of the RBC’s from the PRP and high-spin for separating platelet from Platelet Poor Plasma (PPP). RBC’s in PCs in our study is because of flow of scant quantity of RBC’s in the PCs bag in processing during first separation process after the first spin (soft spin). In hard spin, red blood cells (residual cells) can sediment at the bottom with platelets because of the inappropriate separation of PRP from the red cells as the soft spin leads to the presence of residual red cells in PRP and subsequently there are existing residual red cells in the PCs. The TWBC results showed more TWBC on 1st Day in respect to PCs obtained from Stored WB as compared to the freshly obtained PCs. One of the studies reflected that milieu hold of WB has remarkably higher WBC count after a 12 h hold duration in comparison to 4-8 h hold period [5].
Different result was seen in study done by Dijkstra-Tiekstra et al. [1] which showed no notable differences for TWBC count in PCs obtained from overnight-held WB and the freshly prepared PCs. Few studies showed that overnight stored WB at RT may decrease the risk of bacterial contamination as the WBCs will phagocytose the bacteria [1,7].
Less amount of WBC in the PCs in overnight-held WB may decrease Cytomegalovirus risk transmission, HLA immunization, and febrile reactions [8].
In our study there was no substantial difference in relation to the pH on 1st day, 3rd day and 5th day after overnight storage. In other studies, there are significant differences in pH values, probably because consumption of glucose and formation of lactate by the RBCs in the stored WB. Although reduction in pH for PCs obtained from 24 h stored WB had been seen previously. Naturally there is more of lactate formation from glucose by RBC glycolysis in association with inconsiderable drop in pH in contrast to the WB units which are processed within 8 h [9].
Tiekstra et al. [1] states about variation in metabolism of pH, glucose, sodium had shown best results for freshly prepared PCs in comparison with overnight-held WB. If there is reduction in pH to levels around six (pH 6.0) in stored PCs in plasma results in considerable deprivation of the viability of the platelets. To ensure the platelets produced are viable, the platelets should not have acidic pH and the plasma volumes which have suspended platelets should be appropriate for keeping the pH neutral and it should permit for the gaseous exchange [10].
No bacterial growth observed in PCs in both categories of PCs in this study. In vitro studies done where there is inoculated PCs with bacteria advocated that a marked number of PC had prompt rise of bacterial growth on sixth and seventh storage day. Bacterially contaminated platelet component transfusion can lead to clinically significant events at 1 in 25,000 transfusions [11].
Other ways of contamination by bacteria in PCs can also result due to donor bacteraemia and during collection by the skin flora or while processing or during separation, seal leakage or collection bags having micro puncture. Preparation is done in closed and sterile system enables these studies to ensure a product with decreased bacterial contamination risk. In these other parameters such as pO2, CHCO3 (P), Sodium, Potassium, Calcium, Chloride, LDH, and Glucose were analyzed. Platelets obtained from platelets from overnight held WB had more sodium, calcium, chloride, LDH, glucose in contrast to freshly obtained PCs. More studies are required to evaluate these parameters for more scientific research [2].
CONCLUSION
PRP-obtained PCs from overnight WB have notable differences in vitro variables as compared to freshly processed WB. As a whole, this study set forth that the quality (in vitro) of PRP-obtained PCs, obtained from stored WB kept at RT is at minimum with the quality required of PCs for transfusion in patients having thrombocytopenia. There is sparse data available in vivo, but this also advocates that stored WB (for 24 h) is not disadvantageous for PCs quality. In upcoming research studies, functionality of platelets can be appraised on the PCs obtained from stored WB by performing functional status test of platelets. There is need of collecting more in vivo and in vitro data at the time of execution of overnight-held of WB during production of PRP-derived PCs. To conclude the choice of usage of overnight WB at room temperature must be inclusive of the plasma quality and RBC. Variables show wider standard deviation in our study, so more standardization is needed for PCs processing [11].
- Tiekstra MJ, Meer PF, Cardigan R, Devine D, Prowse C, et al. (2011) Platelet concentrates from fresh or overnight-stored blood, an international study. Transfusion 1: 38-44.
- Shabani NRM, Baqir HS (2014) Quality assessment of platelet concentrates prepared after whole blood overnight storage. J Med Biol Eng 3: 87-92.
- Kasim HS, Hassan FM, Lim V (2016) Assessment of platelet concentrate prepared from fresh and overnight whole blood. AJPS 9: 16-20.
- Moroff G, AuBuchon JP, Pickard C, Whitley CP, Heaton WA, et al. (2011) Evaluation of the properties of components prepared and stored after holding of whole blood units for 8 and 24 hours at ambient temperature. Transfusion 51: 7-14.
- Thomas S (2010) Ambient overnight hold of whole blood prior to the manufacture of blood components. Transfus Med 20: 361-368.
- Meer PF, Cancelas JA, Cardigan R, Devine DV, Gulliksson H, et al. (2011) Evaluation of overnight hold of whole blood at room temperature before component processing: Effect of red blood cell (RBC) additive solutions on in vitro RBC measures. Transfusion 51: 15-24.
- Meer PF, Cancelas JA, Vassallo RR, Rugg N, Einarson M, et al. (2011) Evaluation of the overnight hold of whole blood at room temperature, before component processing: platelets (PLTs) from PLT-rich plasma. Transfusion 51: 45-95.
- Eggen JD, Schrijver JG, Bins M (2001) WBC content of platelet concentrates prepared by the buffy coat method using different processing procedures and storage solutions. Transfusion 41: 1378-1383.
- Gulliksson H, Meer PF (2009) Storage of whole blood overnight in different blood bags preceding preparation of blood components: In vitro effects on red blood cells. Blood Transfusion 7: 210-215.
- Singh RP, Marwaha N, Malhotra P, Dash S (2009) Quality assessment of platelet concentrates prepared by platelet rich plasma-plate concentrate, buffy coat poor-platelet concentrate (BC-PC) and apheresis-PC methods. Asian J Transf Sci 3: 86-94.
- Blajchman MA, Beckers EA, Dickmeiss E, Lin L, Moore G, et al. (2005) Bacterial detection of platelets: Current problems and possible resolutions. Transfus Med 19: 259-272.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- BioMed Research Journal (ISSN:2578-8892)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Journal of Pathology and Toxicology Research
- International Journal of Diabetes (ISSN: 2644-3031)
- Chemotherapy Research Journal (ISSN:2642-0236)
- Journal of Rheumatology Research (ISSN:2641-6999)
- Advance Research on Alzheimers and Parkinsons Disease


