Research Article
Frequency and Association of Metastasis among Morphological Types of Breast Cancer
5058
Views & Citations4058
Likes & Shares
Aims: Objective of study was to observe the frequency of breast cancer metastasis among different morphological subtypes passing through different stages of breast cancer.
Introduction: Spread of cancer tissues from site of origin to local or distant areas is not simple. It is a complex process which involves different chemical reactions and systems of body. Simultaneously system and reactions start and series of continuous chemical reactions proceed. There are many morphological sub types but considerable types are Ductal, Papillary, Medullary, Lobular and Tubular. Breast ultrasound is important and earliest technique used to detect abnormal findings in breast either after history, physical exam or mammogram. Ultrasound. X-rays and C.T scan are cheaper easily available techniques to search metastasis of cancer. Bone scan is a cheaper modality for diagnosis bone metastasis or primary bone problems and widely used to find out bone metastasis. It is found positive in some non-malignant conditions, for that reason it is less specific but sensitive test.
Methods: Ultrasound of breast and abdominal organs had been done to detect primary tumor inside breast and secondary spread of cancer to liver. X-ray and C.T scan had been done to detect pulmonary and cerebral metastasis. Bone scan had been done to detect bone metastasis. Obtain tissues via core needle biopsy dipped in Formalin were fixed on slide with the Hematoxylin dye then histopathology had been done for morphological typing, grading and TNM staging.
Result: Distribution of breast cancer patients passing through different stages showed maximum valid and cumulative percentage of patients with stage 4, n=100 respectively. Stage 1 related cases were least in frequency and percentage. Frequency of auxiliary nodal metastasis was noted in majority of cases n=104 and peripheral lymph nodes (clavicular or contra lateral mammary lymph nodes) involvement were least in number n=18. Frequency and percentages of liver and lung metastasis were low while lowest to cerebral metastasis. Metastasis was low in frequency and non-metastatic cases were more. Over all lungs metastasis found predominant. Commonest type was found in the study was infiltrative ductal carcinoma. Papillary is at second number and infiltrative lobular carcinoma is at third number. Parametric and non-parametric calculation showed direct association among TM staging and metastasis to different organs. Results were significant. Distribution was symmetrical and lightly tailed.
Conclusions: Commonest type was found in the study was infiltrative ductal carcinoma. Parametric and non- parametric calculation showed direct association among TM staging and metastasis to different organs. Inverse relation was found with nodal metastasis. Results were significant. Distribution was symmetrical and lightly tailed. Maximum cases were passing through IIB and IIIB. Least cases were of IIA. Metastasis to lung was found predominant among all stages.
Keywords: Breast cancer, Stages, Morphological types, Distribution
INTRODUCTION
Spread of cancer tissues from site of origin to local or distant areas is not simple. It is a complex process which involves different chemical reactions and systems of body. Together system and reactions take part and series of continuous chemical process starts. NOTCH3 Receptors are found involve in conversion of epithelial cells in to mesenchymal cells through a signaling pathway. Mesenchymal stem cells can proliferate continuously and can spread easily though circulation. There are four notch receptors and all are autonomous receptors. Signaling from this autonomous system promotes oncogenesis and metastasis. Still more research is needed to prove this cascade [1].
Types of breast cancer depend upon cells from where they originate.
In situ:
Ductal: Replacement of normal ductal epithelial tissues from uncontrolled replicating cells which are limited up to the layer of origin and noninvasive in nature are referred as ductal carcinoma in situ. Carcinoma in situ is commonest breast cancer type diagnosed during the period of 2008-2012.
Lobular: It is comparatively less common type and diagnosed only 13% during the period of 2008-2012. Its pattern in growth is toward lumen of lobules. It may indicate the development of invasive cancer in future.
Mixed: some noninvasive tumors show mixed properties of ductal and lobular cancers or may have unknown origin.
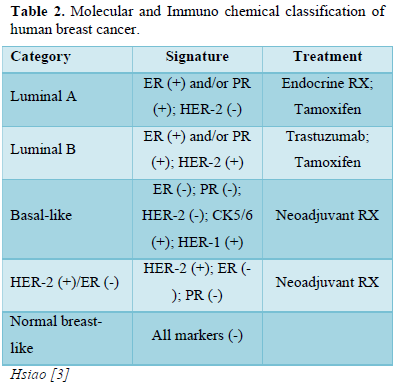
Invasive: It is also common variety. Cancer cells break their fence and disseminate in to neighboring cells. Prognosis of invasive cancer depend upon its staging [2]. Depending upon the morphological and molecular type of Breast cancer different hormonal receptors over express on tissues. There are 5- common categories based on the expression of hormone receptors like estrogen receptor (ER), progesterone receptor (ER), human epidermal growth factor receptor-1 and 2(HER-1 and 2), cytokeratins 5/6 (CK 5/6) (Tables 1 & 2).
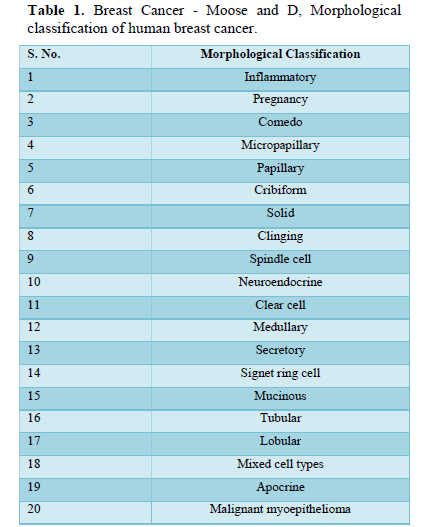


It is evident that ultrasound is more successful to evaluate breast cancer but still mammography is thought to be Breast ultrasound is important and earliest technique used to detect abnormal findings in breast either after history, physical exam or mammogram gold standard as a common tool for the detection of breast abnormalities, especially in dense breast tissues [4].
Computer tomography (CT)
A CT scan (also referred as CAT scan or computerized tomography scan) is a type of X-ray technique that shows the image of organs in form of slices at different planes. For the procedure patient lie on a moving table which under- go through X-ray machine and machine take the cross-section areas of organs in form of slices to show them clearly. Sometimes contrast enhanced materials are used to make the image quality better. Contrast dye can be taken orally or in infusion form then after few hours x-ray of body are taken. An attached computer system with machine compiled these images and creates complete picture. C.T scan also used for the evaluation of chest wall invasion by cancerous tissues. Bone scan is a cheaper modality for diagnosis bone metastasis or primary bone problems and widely used to find out bone metastasis. It is found positive in some non-malignant conditions [5-7], for that reason it is less specific but sensitive test [5-14]. Several studies favor the importance and superiority of MRI [15-17] and CT scan [16] over the bone scan in the diagnosis of metastases (including bone metastases as well as lymph node and visceral involvement). Instead of faster and more sensitive available imaging tests like MRI or PET scan, bone scan is still a common and early suggested scan by physician to rule out primary and secondary bone pathologies because of its easy availability and low cost [10,18].
AIMS
Objective of study was to observe the frequency of breast cancer metastasis among different morphological subtypes passing through different stages of breast cancer.
METHODOLOGY
Cross Sectional Observational Study
Selection of Patients
The present study was carried out on 250 patients taken from Jinnah Post Graduate Medical Centre, (department of oncology) randomly selected from community. Only diagnosed cases of breast cancer were selected for the study. Included patients were adult females from 18 years to onward, premenopausal, peri-menopausal and postmenopausal. Cases of all four stages of cancer, married, unmarried, having different body mass index. Consent form was described orally to every patient and signature was taken. Study design was approved by Board of Advance Studies, University of Karachi and ethical committee of Jinnah Post Graduate Medical Centre.
Collection of samples: Trained staff was hired for sample collection. Sterilized materials were used.
Inclusion Criteria
Clinically and histo-pathologically diagnosed cases of breast cancer were selected for the study.
Exclusion Criteria
Male patients, Patients suffering from primary Bone diseases, Diabetes, Hepatobiliary disease and previous history of any other type of malignancy were excluded from the study. Collections of tumor tissues for histopathology (Formalin-fixed paraffin-embedded tissue specimens). Tissues were examined for tumor characteristics at chromosome and molecular level at JPMC and BMSI. Ultrasound of abdominal organs had been done to detect secondary spread of cancer to liver. X-ray and C.T scan had been done to detect pulmonary metastasis. C.T scan of brain had been done to detect cerebral metastasis.
Statistics
For statistical analysis, software SPSS was used. Frequency tables, Pie chart and histograms are used to describe distribution, symmetry and tailed of qualitative variables in terms of mean, median, standard deviation percentage and standard error of Skewness / Kurtosis. Two tailed ANOVA test was used to check the statistical substantial variance among group means. Reliability test was applied. Crohnbach Alpha is degree of inner reliability and shows how strictly variables are associated. Regression correlation analysis was applied to characterize the association and strength of association via Pearson parametric calculation and Spears on rho non-parametric calculation among quantitative variables. Regression is defined in terms of regression coefficient (r or s), square of regression coefficient (r2) and adjusted (r).2 A value of P< 0.05 was considered significant, and P< 0.01 was considered as statistically significant.
RESULT
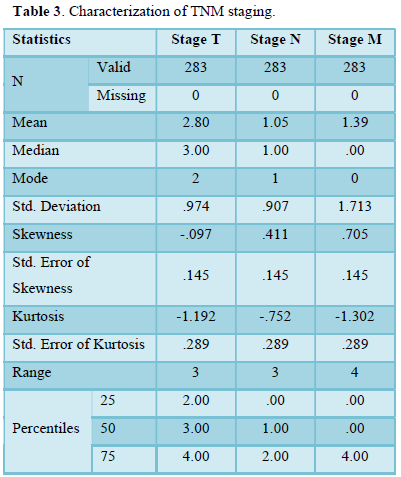
Table 3 shows characterization of TNM staging in term of mean, median and standard deviation of breast cancer population. Further characterization of data includes skewness and kurtosis-Skewness shows a measure of symmetry, or more precisely, the lack of symmetry. Following data is symmetrical. Kurtosis is a measure of whether the data are heavy-tailed or light-tailed relative to a normal distribution.

Frequency Table
Table 4 shows distribution of breast cancer patients passing through different stages in term of frequency, valid and cumulative percentage. Most frequent cases included in study were passing through stage 2, n=102 then stage 4, n=89. Maximum valid and cumulative percentage was of patients with stage 2, n=36 and stage 4, n=100 respectively. Stage 1 related cases were least in frequency n percentage.
Table 5 shows distribution of breast cancer patients with nodal metastasis in term of frequency, valid and cumulative percentage. Frequency of auxiliary nodal metastasis was noted in majority of cases n=104 and peripheral lymph nodes (supra-clavicular or contra lateral mammary lymph nodes) involvement were least in number n=18. Maximum valid and cumulative percentage found in patients with regional and distant nodal involvement n=36 and 100 respectively. Frequency and percentage of patients without nodal metastasis was n= 91 and n= 32 respectively.
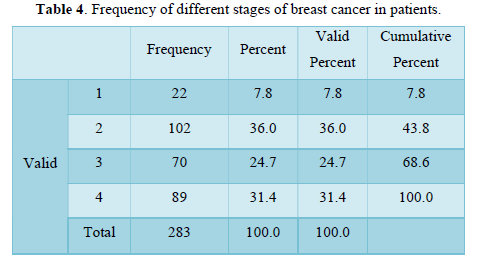
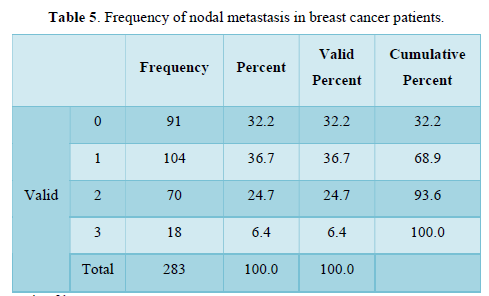


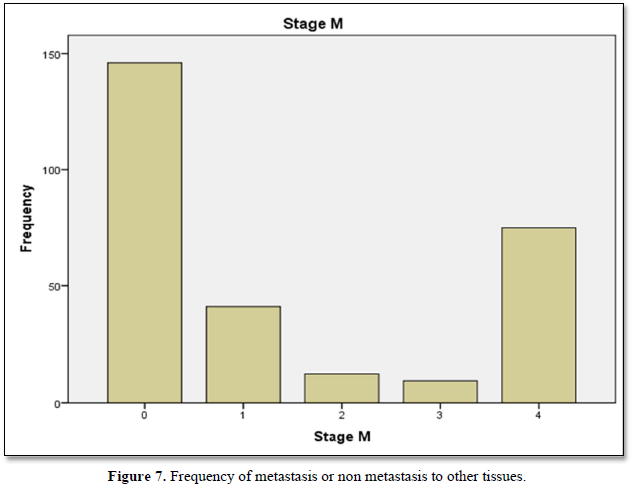
Table 6 shows the metastasis of breast cancer among stage 4 patients in term of frequency n= 75, percentage, valid percentage n=26.5 and cumulative percentage n=100.
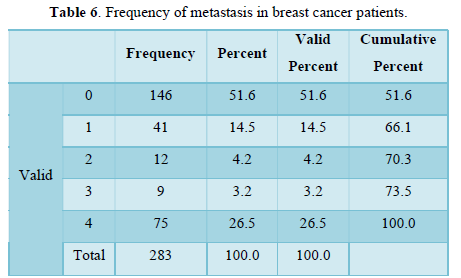

Table 7 is showing frequency n= 217 negative and n= 66 positive. Percentages n= 76 negative and n= 23.3 positive of breast cancer metastasis to liver. Frequency and percentages of liver metastasis are low.
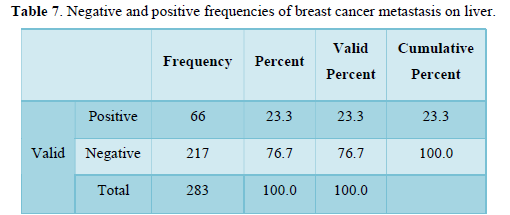

Table 8 shows frequency and percentages of breast cancer metastasis to brain. Frequency n= 284 negative and 34 positive is low. Percentages n= 87.6 negative and n=12 positive. Frequency and percentages of cerebral metastasis are very low.
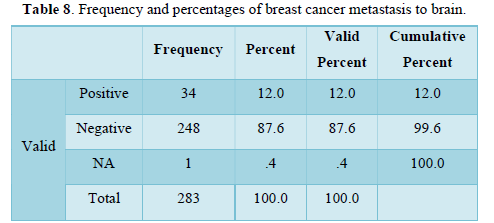

Table 9 shows frequency and percentages of breast cancer metastasis to lungs. Frequency n= 212 negative, 71 positive and percentages n= 74 in negative, n=25 positive, of lung metastasis is low.
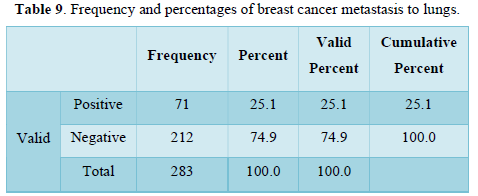

Table 10 shows frequency n=203, 40 and 31 and percentages n= 71, 14, and 11 respectively of different morphological types. Commonest type was found in the study is infiltrative ductal carcinoma. Papillary is at second number and infiltrative lobular carcinoma is at third number.
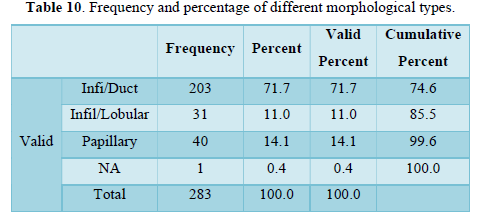

Table 11 shows descriptive analysis of TNM staging and metastatic burden to bones, lungs, liver and brain in terms of mean and standard deviation. Mean of metastasis to liver, bone and lung are approximately equal while slightly higher to brain. Standard deviation is higher for “M” (metastasis) in comparison to T and N. Standard deviation is same for bone, liver and lung metastasis but least for cerebral mets.
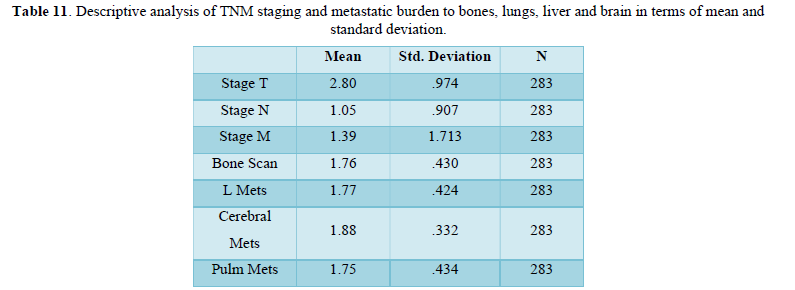

Table 12 shows frequency of metastasis, morphological types of tumors in terms of mean, mode, median and Standard deviation. Symmetry and two tail distributions are also determined via the calculation through standard error of skewness and kurtosis. Mean of metastasis to liver, bone and lungs are approximately equal while slightly higher to brain. Standard deviation is nearly same for bone, liver and lung metastasis but least for cerebral mets.
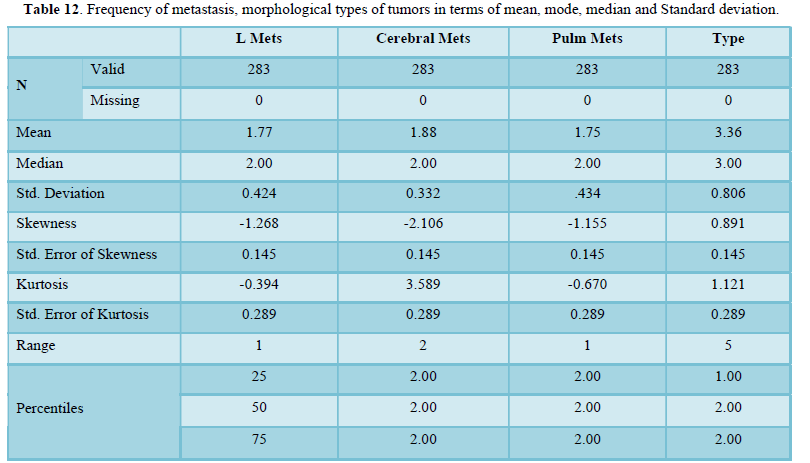

Table 13 shows descriptive analysis of TNM staging and metastatic burden to bones, lungs, liver and brain in terms of mean and Standard deviation. Mean of metastasis to liver, bone and lung are approximately equal while slightly higher to brain. Standard deviation is higher for “M” (metastasis) in comparison to T and N. Standard deviation is same for bone, liver and lung metastasis but least for cerebral mets.
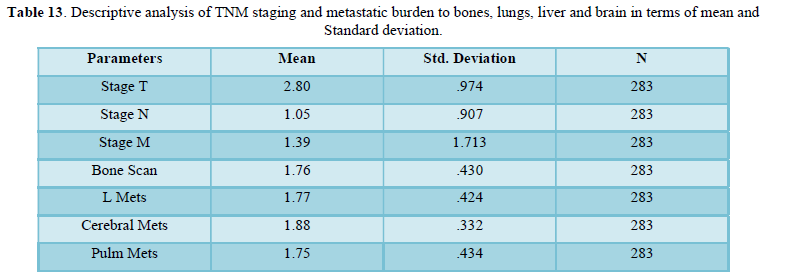

Parametric Correlation
Table 14 parametric correlation shows linear association of TNM staging to metastasis of organs like liver, bone, lungs and brain. Associations among variables are significant.
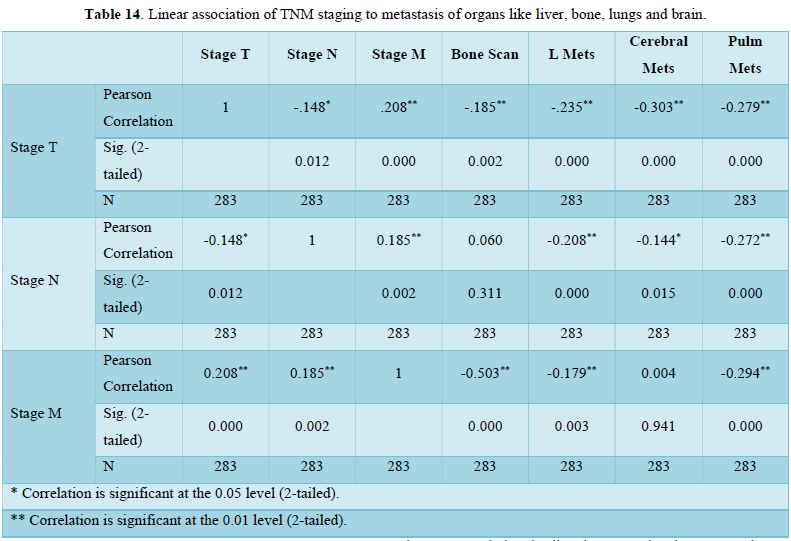

Non-Parametric Correlation
Table 15 shows correlation coefficient of TNM staging and metastatic burden on body organs brain, lungs, liver and bones. Correlation is directly proportional to T, M, bone mets, lung mets and liver mets but inversely related to N and cerebral mets. Correlation of T N M is significant to all mentioned organs in Table 15.
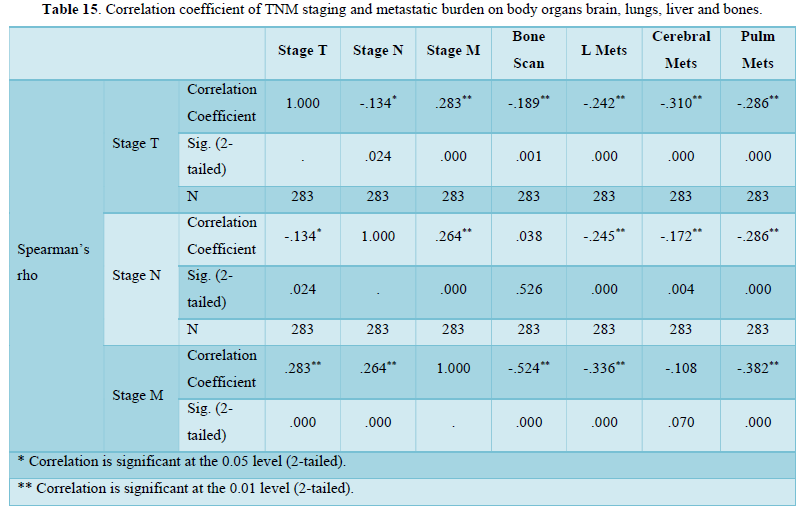

Figure 1 shows distribution of positive and negative cases of liver mets. Maximum cases approximately ¾ are negative for mets.
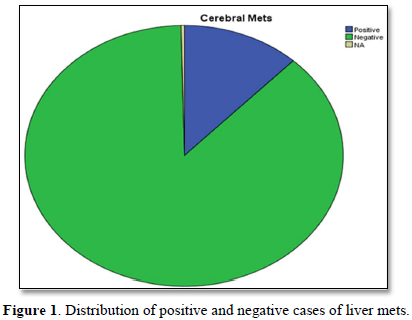

Figure 2 shows distribution of positive and negative cases of cerebral mets. Maximum cases approximately less than 3/4 are negative for mets.
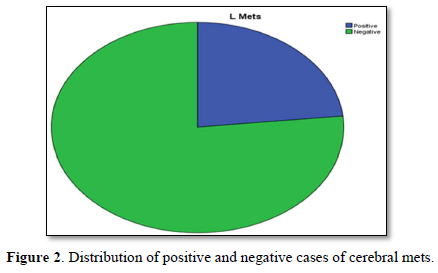

Figure 3 shows distribution of positive and negative cases of cerebral mets. Maximum cases approximately 3/4 are negative for mets.
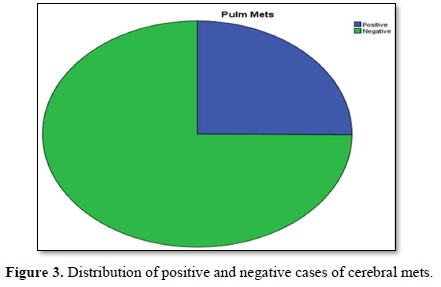

Figure 4 shows distribution of different common morphological types of breast cancer. Maximum cases approximately 3/4 are infiltrative ductal type then papillary approximately less than 1/4. Infiltrative lobular type is at third number and others are least frequent in occurrence.
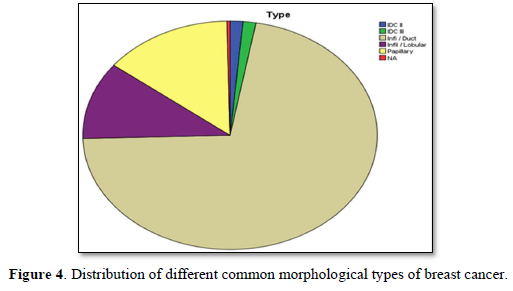

Figure 5 shows distribution of breast cancer among patients passing through different stages. Maximum patients were in stage II then stage IV then stage III. Least cases were of stage.
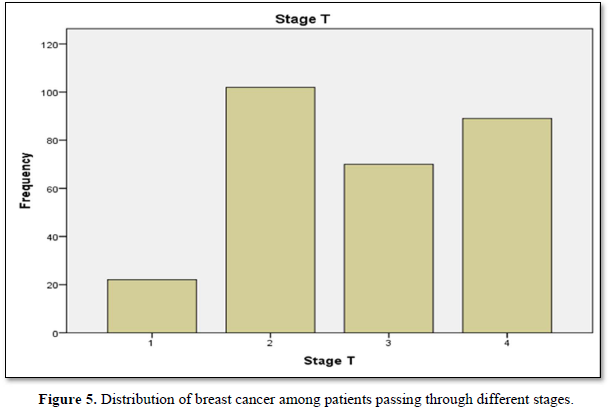

Figure 6 shows frequency bars of nodal metastsasis. Invlovment of ipsilateral mobile nodes are greates, matted or fixed ipsilateral nodes are greater and clavicular or internal mammary nodes are least.
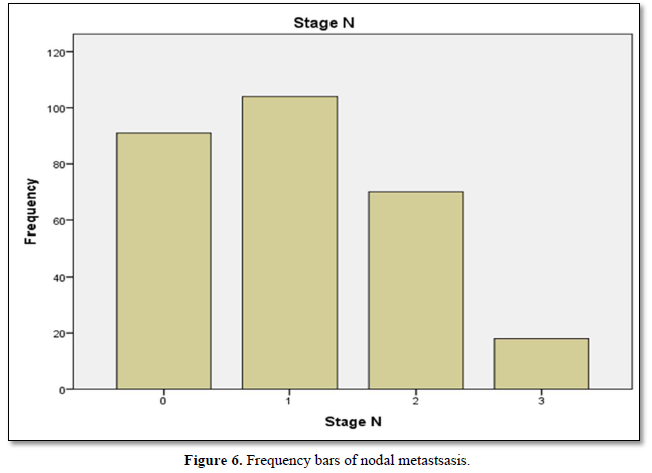

Figure 7 shows frequency of metastasis or non metastasis to other tissues. Nonmetastasis was superior while stage 4 is maximum.

DISCUSSION
Our study shows the characterization of TNM staging in term of mean, median and standard deviation of breast cancer population. Further characterization of data includes skewness and kurtosis. Skewness shows a measure of symmetry, or more precisely, the lack of symmetry. The data of study is symmetrical. Kurtosis is a measure of whether the data are heavy-tailed or light-tailed relative to a normal distribution. Calculation showed data was lightly tailed. Distribution of breast cancer patients passing through different stages showed most frequent cases included in study were passing through stage 2 then stage 4. Maximum valid and cumulative percentage was of patients with stage 2 and stage 4 respectively. Stage 1 related cases were least in frequency and percentage. Distribution of breast cancer patients with nodal metastasis were noted in majority of cases and peripheral lymph nodes (clavicular or contralateral mammary lymph nodes) involvement were least in number. Maximum percentage found in patients with regional and distant nodal involvement was low. Frequency and percentage of patients without nodal metastasis were high. Frequency and percentages of liver metastasis were low. Positive patients for metastasis to liver were less as compare to negative. Frequency of cerebral metastasis was found very low than another site of metastasis. Frequency and percentages of breast cancer metastasis to lungs showed that negative cases were more than positive cases. Commonest type was found in the study was infiltrative ductal carcinoma. Papillary was at second number and infiltrative lobular carcinoma was at third number. Parametric correlation showing linear association of TNM staging to metastasis of organs like liver, bone, lungs and brain. Association among variables is significant. Non-parametric correlation showing correlation coefficient of TNM staging and metastatic burden on body organs brain, lungs, liver and bones. Correlation is directly proportional to T, M, bone mets, lung mets and liver mets but inversely related to N and cerebral mets. Correlation of TNM is significant to all mentioned organs. Frequency of metastasis or non-metastasis to other tissues showed that non-metastasis cases of were more. stage 4 cases were maximum in number. Accurate disease staging is important in decision-making for patients with primary breast cancer, both in treatment planning (locoregional versus systemic therapy) and in establishing the likely prognosis. Determining the presence of metastasis both at presentation and after initial treatment is a key factor in optimal diagnosis and determining ongoing treatment [19-20]. Despite guidelines it is unclear if there is consistency as to the most appropriate initial staging investigations, and therefore the type and timing of staging investigations vary greatly between units. The likelihood of metastatic disease at the time of breast cancer diagnosis is very low 19, so there is no clear evidence to support universal baseline intensive staging, and in fact several studies have suggested that staging in this manner is of limited value [19-25]. The yield of staging investigations is particularly low for patients with small tumors and negative auxiliary nodes. However, many patients continue to undergo extensive staging at the time of diagnosis [26-28]. Over staging can lead to unnecessary resource use (which could be better used to appropriately stage other patients), unnecessary psychological distress [29], and possible delays to treatment. Observations and experiments enhance the confidence for Quality assured practice and facilitates further continuity in field of research [30]. Due to considerable burden of Breast cancer a rational and cost-effective regimen is needed to over-come the current situation. Knowledge and implementation regarding the staging for breast cancer treatment especially in early cases play key role for future planning. To make an accurate staging there must be focused and standardized investigation to classify early cases of breast cancer. For the concerned purpose many international guidelines are available to serve for staging in early cases [31-35]. Other studies showed that common sites of metastatic disease in breast cancer include bone, lung, and liver. Traditionally, staging investigations have therefore included isotope bone scan, chest radiography, and liver ultrasound. All of the staging investigations commonly employed in breast cancer (including CXR, BS, LUS as well as biochemical assays and tumor markers) have been shown to have very low detection rates when used at the time of diagnosis. Schneider [25] found that the overall rate of distant metastasis was only 3.9%, and several papers have reported the detection rates of individual staging investigations. For isotope bone scan this has been published as 0.5-11% [23,25,37,42] for liver ultrasound 0.24-3.3% [23,25,39,43] and for chest radiography 0.2-1.2% [25,37,39,44,45]. Older guidelines, such as the National Comprehensive Cancer Network [35] recommended a comprehensive base- line workup; however, several studies have found that the routine undertaking of staging investigations is unnecessary, and this is now reflected in current guidelines. A large study found that of the 80% patients diagnosed with early breast cancer undergoing baseline BS, skeletal metastasis was only detected in approximately 6%, whilst baseline CXR and LUS had even lower pick-up rates [46]. In a cohort of 781 patients evaluated by Morris et al., 34% underwent a staging bone scan, but only 14.3% patients presented with metastatic disease at any site, and the yield of bone scans was only 15.8% [47]. Importantly in this study, the majority of patients found on staging isotope bone scan to have skeletal metastases were symptomatic, and there was high clinical suspicion in the vast majority of the remainder. This amounted to an incidental bony metastasis rate of only 1% [47]. In contrast, they found that the negative predictive value of normal liver function tests was 97.6%. This suggests that patients should undergo liver biochemistry routinely, particularly as they present much lower cost [47], but our survey would suggest that over 30% of respondents are not using liver function tests routinely.
CONCLUSIONS
Metastasis was low in frequency and non-metastatic cases were more. Commonest type was found in the study was infiltrative ductal carcinoma. Papillary is at second number and infiltrative lobular carcinoma is at third number. Parametric and non-parametric calculation showed direct association among TM staging and metastasis to different organs. Results were significant. Distribution was symmetrical and lightly tailed. Maximum cases were passing through IIB and IIIB. After that cases of stage IIIA were more. Least cases were of IIA. Metastasis to lung was found predominant in all stages.
- Leontovich AA, Jalalirad M, Salisbury JL, Mills L, Haddox CC, et al. (2018) NOTCH3 expression is linked to breast cancer seeding and distant metastasis. Breast Cancer Res 20: 105.
- Alteri R, Bertaut T, Brinton LA, Fedewa S, Freedman RA, et.al (2015) Breast Cancer Facts & Figures. Am Cancer Soc Atlanta, Georgia.
- Hsiao Y-H, Chou M-C, Fowler C, Mason JT, Man Y-G (2010) Breast cancer heterogeneity: Mechanisms, proofs, and implications. J Cancer 1: 6-13.
- Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, et al. (2012) Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA 307: 1394-1404.
- Heidenreich A, Aus G, Bolla M, Joniau S, Matveev VB, et al. (2008) EAU guidelines on prostate cancer. Eur Urol 53: 68-80.
- O’Sullivan JM, Norman RA, Cook GJ, Fisher C, Dearnaley DP (2003) Broadening the criteria for avoiding staging bone scans in prostate cancer: A retrospective study of patients at the Royal Marsden Hospital. BJU Int 92(7): 685-689.
- Lee N, Fawaaz R, Olsson CA, Benson MC, Petrylak DP, et al. (2000) Which patients with newly diagnosed prostate cancer need a radionuclide bone scan? An analysis based on 631 patients. Int J Radiat Oncol Biol Phys 48(5): 1443-1446.
- Lee CT, Oesterling JE (1997) Using prostate-specific antigen to eliminate the staging radionuclide bone scan. Urol Clin North Am 24(2): 389-394.
- McGregor B, Tulloch S, Quinlan F, Lovegrove F (1978) The role of bone is scanning in the assessment of prostatic carcinoma. Br J Urol 50: 178-181.
- Haukaas S, Roervik J, Halvorsen O, Foelling M (1997) When is bone scintigraphy necessary in the assessment of newly diagnosed, untreated prostate cancer? Br J Urol 79: 770-776.
- Pollen J, Gerber K, Ashburn W, Schmidt J (1981) Nuclear bone imaging in metastatic cancer of the prostate. Cancer 47: 2585-2594.
- Hirobe M, Takahashi A, Hisasue S-I, Kitamura H, Kunishima Y, et al. (2007) Bone scanning-who needs it among patients with newly diagnosed prostate cancer? Jpn J Clin Oncol 37: 788-792.
- Rydh A, Tomic R, Tavelin B, Hietala S, Damber J (1999) Predictive value of prostate-specific antigen, tumor stage and tumor grade for the outcome of bone scintigraphy patients with newly diagnosed prostate cancer. Scand J Urol Nephrol 33: 89-93.
- Gleave M, Coupland D, Drachenberg D (1996) Ability of serum prostate-specific antigen levels to predict normal bone scans in patients with newly diagnosed prostate cancer. Urology 47: 708-712.
- Lecouvet F, Geukens D, Stainier A, Jamar F, Jamart J, et al. (2007) Magnetic resonance imaging of the axial skeleton for detecting bone metastases in patients with high-risk prostate cancer: Diagnostic and cost-effectiveness and comparison with current detection strategies. J Clin Oncol 25: 3281-3287.
- Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT (2007) Imaging prostate cancer: A multidisciplinary perspective. Radiology 243: 28-53.
- Traill ZC, Talbot D, Golding S, Gleeson FV (1999) Magnetic resonance imaging versus radionuclide scintigraphy in screening for bone metastases. Clin Radiol 54: 448-451.
- Heidenreich A, Albers P, Classen J, Graefen M, Gschwend J, et al. (2010) Imaging studies in metastatic urogenital cancer patients undergoing systemic therapy: Recommendations of a multidisciplinary consensus meeting of the association of urological oncology of the German cancer society. Urol Int 85: 1-10.
- Myers RE, Johnston M, Pritchar K, Levine M, Oliver T, et al. (2001) Baseline staging tests in primary breast cancer: A practice guideline. CMAJ 164: 1439-1444.
- Ravaioli A, Pasini G, Polselli A, Papi M, Tassinari D, et al. (2002) Staging of breast cancer: New recommended standard procedure. Breast Cancer Res Treat 72: 53-60.
- Chen E, Carlson G, Coughlin B, Reed J, Garb J, et al. (2000) Routine chest roentgenography is unnecessary in the work-up of stage I and II breast cancer. J Clin Oncol 18: 3503-3506.
- Dillman R, Chico S (2000) Radiologic tests after a new diagnosis of breast cancer. Eff Clin Pract 3: 1-6.
- Samant R, Ganguly P (1999) Staging investigations in patients with breast cancer: The role of bone scans and liver imaging. Arch Surg 134: 551-554.
- Gerber B, Seitz E, Müller H, Krause A, Reimer T, et al. (2003) Preoperative screening for metastatic disease is not indicated in patients with primary breast cancer and no clinical signs of tumor spread. Breast Cancer Res Treat 82: 29-37.
- Schneider C, Fehr MK, Steiner RA, Hagen D, Haller U, et al. (2003) Frequency and distribution pattern of distant metastases in breast cancer patients at the time of primary presentation. Arch Gynecol Obstet 269: 9-12.
- Khansur T, Haick A, Patel B, Balducci L, Vance R, et al. (1987) Evaluation of bone scan as a screening work-up in primary and local-regional recurrence of breast cancer. Am J Clin Oncol 10: 167-170.
- Nomura Y, Kondo H, Yamagata J, Kanda K, Takenaka K, et al. (1978) Evaluation of liver and bone scanning in patients with early breast cancer, based on results obtained from more advanced cancer patients. Eur J Cancer 14: 1129-1136.
- Wiener SN, Sachs SH (1978) An assessment of routine liver scanning in patients with breast cancer. Arch Surg 113: 126-127.
- Han JY, Kim J-H, Yoon HJ, Shim M, McTavish FM, et al. (2012) Social and psychological determinants of levels of engagement with an online breast cancer support group: Posters, lurkers, and nonusers. J Health Commun 17: 356-371.
- Hogeveen SE, Han D, Trudeau-Tavara S, Buck J, Brezden-Masley CB, et al. (2012) Comparison of international breast cancer guidelines: Are we globally consistent? Cancer guideline Agreement. Curr Oncol 19: 184-190.
- National Collaborating Centre for Cancer (NICE) (2009) CG80-Early and locally advanced breast cancer: Diagnosis and Treatment. Full guideline.
- Association of Breast Surgery at BASO (2009) Surgical guidelines for the management of breast cancer. E JSO.
- Cancer Care Ontario (CCO) (2011) Members of the breast cancer disease site group. Baseline staging tests in primary breast cancer. Evidence-based guideline 1-14 Version 2.
- National Comprehensive Cancer Network (NCCN) (2012) Breast Cancer Version 2.
- Carlson R, Goldstein J, Gradishar W (1996) NCCN breast cancer practice guidelines. The National Comprehensive Cancer Network. Oncology 10: 47-75.
- Lee Y (1981) Bone scanning in patients with early breast carcinoma: Should it be a routine staging procedure? Cancer 47: 486-495.
- Monypenny IJ, Grieve RJ, Howell A, Morrison JM (1984) The value of serial bone scanning in operable breast cancer. Br J Surg 71: 466-468.
- Ciatto S, Pacini P, Azzini V, Neri A, Jannini A, et al. (1988) Preoperative staging of primary breast cancer. A multicenter study. Cancer 61: 1038-1040.
- Piffer S, Amichetti M, Valentini A (1988) Skeletal scintigraphy and physical examination in the staging of early breast cancer. Act Oncol 27: 21-24.
- Cox MR, Gilliland R, Odling-Smee GW, Spence RA (1992) An evaluation of radionuclide bone scanning and liver ultra-sonography for staging breast cancer. Aust N Z J Surg 62: 550-555.
- Alcazar J, Rolle A, Murcia J, Baldonado C, Lopez Garcia G (1995) Bone scanning in preoperative staging of early breast cancer patients: should it be a routine procedure? Breast Dis 8: 7-11.
- Bruneton JN, Balu-Maestro C, Raffaelli C, Mourou MY, Cambon P, et al. (1996) Indications for hepatic ultra-sonography in breast cancer staging and follow-up. Breast Cancer Res Treat 37: 115-121.
- Vestergaard A, Herrstedt J, Thomsen HS, Dombernowsky P, Zedeler K (1989) The value of yearly chest X-ray in patients with stage I breast cancer. Eur J Cancer Clin Oncol 25: 687-689.
- Davis DA, Taylor-Vaisey A (1997) Translating guidelines into practice. A systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. CMAJ 157: 408-416.
- Puglisi F, Follador A, Minisini AM, Cardellino GG, Russo S, et al. (2005) Baseline staging tests after a new diagnosis of breast cancer: Further evidence of their limited indications. Ann Oncol 16: 263-266.
- Morris PG, O'Connor M, O'Rafferty C, Sheikh R, Gray J, et al. (2009) The excessive cost of baseline diagnostic imaging in early breast cancer. Ir Med J 102: 149-152.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Advance Research on Endocrinology and Metabolism (ISSN: 2689-8209)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Advance Research on Alzheimers and Parkinsons Disease
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Otolaryngology and Neurotology Research(ISSN:2641-6956)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)






