4668
Views & Citations3668
Likes & Shares
Preeclampsia (PE) is one of the most serious complications of pregnancy. Disturbances in immune regulation are one of the most common reasons that negatively influences the gestation process and play an important role in pathogenesis of PE We hypothesize that alteration of serum natural autoantibodies’ level, may be associated with PE. Natural autoantibodies were measured by ELI-P complex in maternal sera from preeclamptic subjects in comparison to normal pregnancies. The corresponding peaks of immunoreactivity were selectively affected in approximately 89% of preeclampsia and 12 % control pregnant (p<0,001). This study confirms the high level of immunological activation in patients with PE. The presence of autoantibodies implies that autoimmunity might have a causal role in the pathogenesis of PE.
At the beginning of the 21st century, the term ‘Immunculus’ has been proposed by Poletaev and Osipenko for describtio of the global system (network) of constitutively expressed natural autoantibodies (na-Ab) interacting specifically with different self-antigens [19]. In healthy individuals the repertoires of each na-Ab are surprisingly constant and characterized by minimal individual quantitative variations [20].
Our study aims to evaluate the IgG autoantibodies against different autoantigens in the serum of patients with PE by ELI-P complex method and its usefulness and application in daily practice.
MATERIALS AND METHODS
The Study Design
This study was performed at the University General Hospital of Alexandroupolis and Democritus University of Thrace in Alexandroupolis, Greece. The study protocol was approved by the local committee of ethics and deontology in accordance with the Declaration of Helsinki (Ethics Committee identification code: 941).
Study Population
This study included 92 pregnant women persons mean age 32.38±1.59 yrs. All patients were divided into two groups: Preeclampsia n=57, and control n=35. Serum and urine samples were collected on admission to the hospital. The period of sampling was from June 2014 to May 2020.
Biochemical analysis
Biochemical tests were performed by automated biochemical analyzer. To estimate the renal function sCr was measured using an automated biochemical analyzer, and the estimated glomerular filtration rate (e-GFR) was calculated using CKD EPI formula.
ELI -P complex ELISA
Median individual immune reactivity (MIR) and profiles of individual immune reactivity (relative content) of the corresponding autoantibodies were detected and analyzed in the blood serum samples as described previously by Poletaev AB [21]. ELI-P-Complex method is intended for measuring the profiles of specific serum immune reactivity (IR) which depends on a serum content of twelve IgG a-Abs to Chorionic Gonadotropin (hCG), dsDNA, β2-Glycoprotein I, Fc-fragment of IgG (rheumatoid factor), collagen II, insulin, thyroglobulin, proteins of S100 family, Spr-06-antigen of sperm membranes, TrM-03-antigen of platelet membranes, ANCA, KiM-05-antigen of kidney cell membranes. Selective increase in serum immunoreactivity with certain antigens from +10% and above or selective decrease from -15% and below (from the individual average level of the reaction) were considered as abnormal peaks.
RESULTS
The systolic as well as diastolic pressure was increased in PE in a statistically significant manner 160.14±7.6 vs 128.3±7.1, and 92.6±1.4 vs 74.7±7.5, respectively. The characteristics and laboratory findings of the study population are presented in Table 1.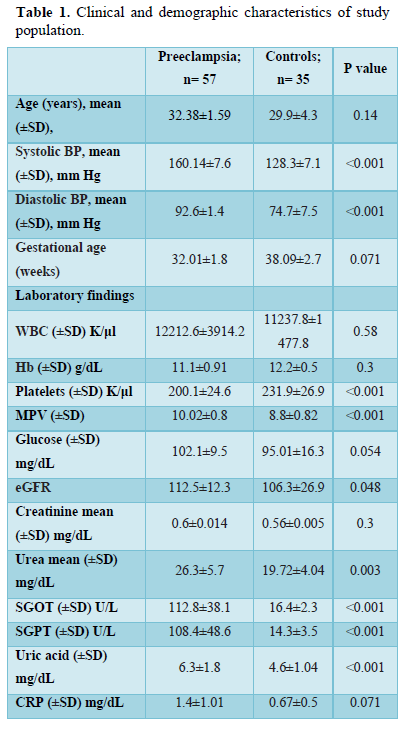
The corresponding peaks of immunoreactivity were selectively affected in approximately 89% of preeclampsia and 12 % control pregnant (p<0,001). More specifically, IgG autoantibodies to chorionic gonadotropin (hCG) were found to be elevated in 8 (14%) patients of PE group vs. 1 (2.5%), anti-β2-Glycoprotein I a-Abs were decreased in 16 (28,1%) PE patients, while were found to be elevated in 3(5,2%). Antibodies to collagen II were decreased in 38 (66,6%) patients vs. 4 (11,4%) in control groups. Antibodies to insulin, were elevated in 26 (45,6%) vs. 6 (17,1%). Moreover, in our study the increasing level of anti-insulin antibodies were correlated with highest glucose level (Figure 1). Antibodies to proteins of S100 family were increased in 37 (64.9%) vs. 7 (20%). Anti-platelet antibodies, TrM-03-antigen of platelet membranes, were increased in patients with PE, who develop HELP syndrome. We also found that serum level of anti -KiM-05-antigen of kidney cell membranes were inversely associated with preeclampsia.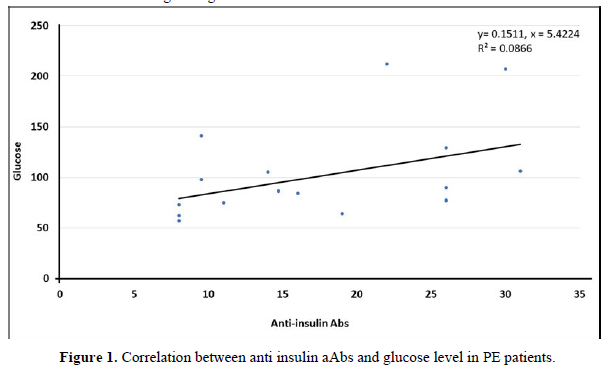
DISCUSSION
For a long time, the elevated levels of autoantibodies have been associated exclusively with the pathogenesis-diagnosis of autoimmune diseases. Nevertheless, it has been recognized that the level of autoantibodies can be increased in other diseases, not within the spectrum of autoimmunity, like asthma, stroke, cognitive dysfunction in children and adults etc. [22,23].
We report the laboratory findings of a cohort of 57 patients with PE, from whom 89% have been affected tests for autoantibodies. The diagnosis of PE is based on clinical criteria, while laboratory tests show wide variability within assays, making diagnosis quite difficult.
In this study, we found that the mean individual immune reactivity in PE was significantly higher than in the control subjects. Our results are in line with previously reported data [21,24].
We found that antibodies to ds DNA was inversely associated with preeclampsia. It is worth noting that regarding antibodies to ds DNA it is several contradictory data: in several works, a clear increase in their concentration was noted in patients with PE, while, on the other studies its decrease [24,25].
Antibodies to collagen were decreased in PE group in comparison to controls (38/66,6% vs. 4/11,4%). A possible explanation for this might be that a-Abs cannot be detected because they form complexes with antigens which are in excess in patients with PE. Nikolov [26] report that serum levels of collagen were elevated in early-onset preeclampsia [26]. Antibodies to insulin were increased in 21 (36.8%) patients PE. It is well documented that gestational diabetes mellitus consists of risk factor for develop of PE [27,28]. This may be a potential explanation for the presence of antibodies to insulin in patients with PE. Moreover, in our study the increasing level of anti-insulin antibodies were correlated with highest glucose level (Figure 1).
We also found that serum levels of anti S100 a-Abs were associated with preeclampsia. S100 proteins regulate various cells' signaling pathways. Studies report that increased level of S100 proteins is found to be associated with pregnancy disorders such as preeclampsia or early pregnancy loss. [29,30].
Additionally, serum levels of anti -KiM-05-antigen of kidney cell membranes was inversely associated with preeclampsia. It is well documented that preeclampsia is characterized by kidney damage, and consequence proteinuria. The production of kidney specific antigens may be triggered by kidney damage. Moreover, the new environment during pregnancy (the process of placentation) triggers physiological changes that modulate kidney function, with intention to control extracellular volume and acid-base balance during the pregnancy. This bidirectional communication between placenta and kidney signifies that changes or dysfunction of one organ influence over the others [31]. In accordance with the above, it is possible that a-Abs cannot be detected because they form complexes with antigens which are in excess in patients with PE.
In conclusion, changes in the production of autoantibodies play an important homeostatic role in various disorders as in preeclampsia. The study of autoantibodies provides optimization of the differential diagnosis of preeclampsia, makes it possible to predict the development of complications in preeclampsia such as kidney damage or hyperinsulinemia, and might significantly reduce maternal and infant morbidity and mortality.
ACKNOWLEDGEMENTS
We would like to thank the Medical Research Centre (MRC), ‘Immunculus’ for providing with the ELI -P kit.
- No authors’ listed (2020) Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet Gynecol 135(6): e237-e260.
- Han M, Liu D, Zeb S, Li C, Tong M, et al. (2019) Are maternal and neonatal outcomes different in placental abruption between women with and without preeclampsia? Placenta 85: 69-73.
- Wisner K (2019) Gestational hypertension and preeclampsia. MCN Am J Matern Nurs 44(3): 170.
- Lei T, Qiu T, Liao W, Li K, Lai X (2021) Proteinuria may be an indicator of adverse pregnancy outcomes in patients with preeclampsia: A retrospective study. Reprod Biol Endocrinol 19(1): 71.
- Jung E, Romero R, Yeo L, Gomez-Lopez N, Chaemsaithong P, et al. (2022) The etiology of preeclampsia. Am J Obstet Gynecol 226(2S): S844-S866.
- MacDonald TM, Walker SP, Hannan J, Tong S, Kaitu'u-Lino TJ (2022) Clinical tools and biomarkers to predict preeclampsia. EBioMedicine 75: 103780.
- Burton GJ, Redman CW, Roberts JM, Moffett A (2019) Pre-eclampsia: Pathophysiology and clinical implications. BMJ 366: l2381.
- Hecht JL, Zsengeller ZK, Spiel M, Karumanchi A, Rosen S (2016) Revisiting decidual vasculopathy. Placenta 42: 37-43.
- Tannetta D, Masliukaite I, Vatish M, Redman C, Sargent I (2017) Update of syncytiotrophoblast derived extracellular vesicles in normal pregnancy and preeclampsia. J Reprod Immunol 119: 98-106.
- Hubel CA (1999) Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med 222: 222-235.
- Albogami SM, Al-Kuraishy HM, Al-Maiahy TJ, Al-Buhadily AK, Al-Gareeb AI, et al. (2022) Hypoxia-Inducible Factor 1 and Preeclampsia: A New Perspective. Curr Hypertens Rep 24(12): 687-692.
- Bolatai A, He Y, Wu N (2022) Vascular endothelial growth factor and its receptors regulation in gestational diabetes mellitus and eclampsia. J Transl Med 20(1): 400.
- Vishnyakova P, Kuznetsova M, Poltavets A, Fomina M, Kiseleva V, et al. (2022) Distinct gene expression patterns for CD14++ and CD16++ monocytes in preeclampsia. Sci Rep 12(1): 15469.
- Giaglis S, Stoikou M, Grimolizzi F, Subramanian BY, van Breda SV, et al. (2016) Neutrophil migration into the placenta: Good, bad or deadly? Cell Adh Migr 10(1-2): 208-225.
- Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S (2005) Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol 66: 1146-1154.
- Hahn S, Giaglis S, Hoesli I, Hasler P (2012) Neutrophil NETs in reproduction: from infertility to preeclampsia and the possibility of fetal loss. Front Immunol 3: 362.
- Mora-Palazuelos C, Bermúdez M, Aguilar-Medina M, Ramos-Payan R, Ayala-Ham A, et al. (2022) Cytokine-polymorphisms associated with Preeclampsia: A review. Medicine (Baltimore) 101(39): e30870.
- Fong FM, Sahemey MK, Hamedi G, Eyitayo R, Yates D, et al. (2014) Maternal genotype and severe preeclampsia: A HuGE review. Am J Epidemiol 180(4): 335-345.
- Poletaev AB, Osipenko L (2003) General network of natural autoantibodies as immunological homunculus (Immunculus). Autoimmun Rev 2(5): 264-271.
- Poletaev A (2012) Maternal Immunity, Pregnancy and Child’s Health. In: Sifakis, S. and Vrachnis, N., Eds., From Preconception to Postpartum, InTech, Rijeka, pp: 41-56.
- Poletaev AB, Maltseva LI, Zamaleeva RS, Nukhnin MA, Osipenko LG (2007) Application of ELI-P-Complex Method in Clinical Obstetrics. Am J Reprod Immunol 57(4): 294-301.
- Poletaev AB (2008) Immunophysiology and Immunopathology. MIA Press, Moscow.
- Konstantinidis TG, Tsigalou C, Bisiklis A, Romanidou G, Konstantinidou E, et al. (2012) Autoantibodies in patients with asthma: Is there a link between asthma and autoimmunity? Int J Immunol Stud 1(4): 376-338.
- Tsakhilova SG, Sharkovskaya TE, Yakimovich OA, Begizova AM, Malsagova AA (2015) “Eli-P-Complex” Diagnostic Test for Preconception Care in Women with History of Adverse Pregnancy Outcome: A Randomized Multicenter Trial. Adv Reprod Sci 3(4): 81-91.
- Makarov OV, Bogatyrev Yu A, Osipova NA (2012) Significance of autoantibodies in the pathogenesis of preeclampsia. Akusherstvo i ginekologiya/Obstet Gynecol 4-1: 16-21.
- Nikolov AG, Popovski NK, Blazhev AB, Blazheva S. (2021) Comparison of serum levels of collagen type I turnover markers in early-onset preeclampsia and healthy pregnant women. Folia Med (Plovdiv) 63(4): 519-526.
- Kapustin RV, Kopteeva EV, Alekseenkova EN, Tral TG, Tolibova GK, et al. (2021) Placental expression of endoglin, placental growth factor, leptin, and hypoxia-inducible factor-1 in diabetic pregnancy and pre-eclampsia. Gynecol Endocrinol. 37(sup1): 35-39.
- Jorquera G, Fornes R, Cruz G, Thomas-Valdés S (2022) Association of Polyphenols Consumption with Risk for Gestational Diabetes Mellitus and Preeclampsia: A Systematic Review and Meta-Analysis. Antioxidants (Basel) 11(11): 2294.
- Jurewicz E, Filipek A (2022) Ca2+-binding proteins of the S100 family in preeclampsia. Placenta 27: 43-51.
- Nair RR, Khanna A, Singh K (2015) Association of increased S100A8 serum protein with early pregnancy loss. Am J Reprod Immunol 73(2): 91-94.
- Cabarcas-Barbosa O, Capalbo O, Ferrero-Fernández A, Carlos G Musso CG (2022) Kidney-placenta crosstalk in health and disease. Clin Kidney J 15(7): 1284-1289.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Pathology and Toxicology Research
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- Journal of Nursing and Occupational Health (ISSN: 2640-0845)
- Journal of Oral Health and Dentistry (ISSN: 2638-499X)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)



