7875
Views & Citations6875
Likes & Shares
Thymic stromal lymphopoietin (TSLP) is a cytokine produced primarily by activated epithelial cells of the lung, skin and intestine. The foremost property of this cytokine is to condition dendritic cells (DC) to initiate type 2 responses, and consequently to develop a wide range of related disease, including asthma, atopic dermatitis, and allergic responses. However, TSLP is also associated with regulatory and homeostatic processes. The objective of this review is to provide a summary overview of the variety of functions found in this cytokine.
Keywords: Dendritic cells, Thymic stromal lymphopoietin
Abbreviations: DC: conventional dendritic cells; TSLP: thymic stromal lymphopoietin; sTSLP: short isoform TSLP, lTSLP: long isoform TSLP, TSLPR: TSLP receptor; TLR: Toll-like receptor; ACh: acetylcholine; Th: T helper; TFh: T follicular helper; Treg: T regulatory; NKT: Natural killer T; LPS: lipopolysaccharide; TEC: thymic epithelial cells; TECc: cortical thymic epithelial cells; TECm: medullar thymic epithelial cells; RSV: respiratory syncytial virus; HDM: house dust mite; KO: The knockout; HIV: human immunodeficiency virus; IBD: inflammatory bowel diseases; IEC: intestinal epithelial cells; MDC: macrophage-derived chemokine; TARC: thymus and activation-regulated chemokine; UC: ulcerative colitis; CD: Crohn´s disease; BCL- 2: B cell Leukemia/Lymphoma 2; STAT-5: signal transducer and activator of transcription 5; PPAR2: Peroxisome proliferator activated receptor; NF-kB: Nuclear Factor kappa B; TRPA1: transient receptor potential action channel subfamily-member 1/ transient receptor potential ankyrin; RIG-1: retinoic acid inducible gene 1
INTRODUCTION
It is widely recognized that the epithelial lining of several organs, such as skin, lungs and gut, has a fundamental role as a protective barrier against infection and physical or chemical injury [1]. However, the epithelium is no longer considered only as a physical barrier, but it is also the primary one that senses the external environment, working as a key sensor and modulator of the immune response [2]. The thymic stromal lymphopoietin (TSLP) is a cytokine produced by activated lung, skin and gut epithelial cells, inducing the activation of an extensive range of immune and non-immune cells [3,4]. Regarding the immune perspective, the main property of this cytokine is to condition the dendritic cells (DC) to initiate type 2 responses, and consequently a broad array for allergic responses [5,6].
Actually, TSLP is a pleiotropic cytokine belonging to the IL-2 family but it was identified in 1994 [7] as a secreted factor from a mouse thymic stromal cell line; which promote immature B cells [7] and T progenitors [8]. TSLP is a four-helix-bundle cytokine and was first cloned in humans in 2001 [4,9], interestingly close to the gene cluster encoding several Th2-related cytokines [4,10]. The human TSLP gene is located on chromosome 5q22.1 next to the atopic cytokine cluster such as IL-4, IL-5, IL-9, and IL-13 on 5q31
[3,6,9,11,12]. The biological activity of TSLP, in both humans and mice, is mediated by binding to their complex composed by TSLP receptor α chain (RTSLP, chain specific of TSLP, also known as CRLF2; this chain is a member of the hematopoietic receptor family and binds with low affinity to TSLP) and the interleukin 7 receptor-α chain (IL-7Rα), both chains together induce a heteromeric complex of high-affinity [12-14].
There are many different stimuli including some allergens, cytokines, respiratory viruses [1,3,15,16], leading to the production of TSLP in epithelial cells, airway smooth muscle cells, human DCs, and mast cells, etc. [1,3,17,18]. Furthermore, different kind of cells and tissue can respond to TSLP including immune cells (e.g. DCs, ILC2, T and B lymphocytes, natural killer T (NKT), T regulatory cell (Treg.), monocytes, mast cells, macrophages, eosinophils, basophils) and non-immune cells (platelets and sensory neurons, heart, skeletal muscle, kidney and liver) [3,4,12,19], inducing different functions (Table 1).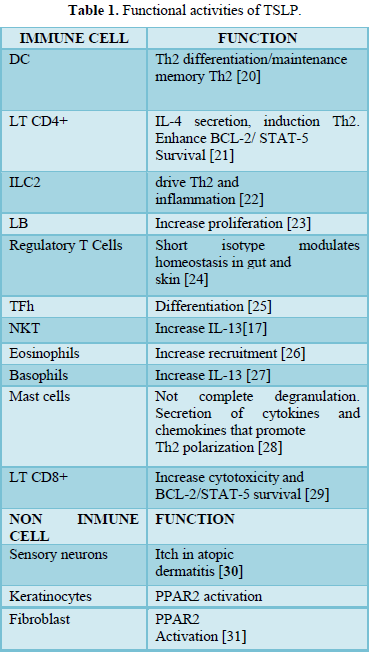
One particularly pertinent reason to develop this topic is that the up regulation of the cytokine itself is closely linked up to the pathogenesis of numerous Th2 related diseases, including asthma, atopic dermatitis and allergic responses [32]. It is reported that the cytokine not only promote Th2 response but also can be associated, with autoimmune disorders [33,34] and finally in recent times has been linked the TSLP to the pathogenesis with different tumors, like breast cancer [35], leukemia lymphocytic acute (ALL) [36], cutaneous T cell lymphomas [37], enhances lung metastasis [38]. Moreover, intratumor Th2-type cell infiltrate correlates with cancer-associated fibroblast TSLP production and reduced survival in pancreatic cancer [39]. On the contrary, TSLP also mediates several immune homeostatic functions in thymic [40], intestinal [41] and trophoblastic cells [42,43].
The objective of this review is to describe the recent advances in the homeostatic and inflammatory mechanisms carried out by the TSLP as modulate of physiology of immune cell mainly DCs.
DC PRIMING BY TSLP: IS RESPONSIBLE FOR PROINFLAMMATORY OR REGULATORY STATUS?
Inflammatory function
Conventional DCs are specialized antigen-presenting cells with a unique ability to activate resting T cells and to direct their differentiation into several effector profiles [5,44-46]. Human TSLP markedly activates and maintains the survival of DCs and Langerhans cells [6,47,48]. In addition, TSLP-conditioned DC up regulated the costimulatory molecules CD40, CD80, CD86 and OX40L, and produces high levels of IL-8 IL-15, Eotaxin2, thymus and activation-regulated chemokine (TARC/CCL17) and macrophage-derived chemokine (MDC/CCL22) [6,49]. Moreover, naive allogeneic T cells that were cocultured with TSLP-conditioned DC acquired an inflammatory Th2-like phenotype with production of IL-4, IL-5, IL-13, and TNF-α but not IL-10 [50,51]. Likewise, it seems that TSLP induces human myeloid DC to express OX40L (the TNF superfamily protein) [49], which induce the generation of inflammatory Th2 cells. The relevance of the OX40L molecule is clearly reflected in the initiation of the Th2 response which, independently of IL-4, depends on the interaction of OX40 with OX40L expressed in activated naïve T and DC cells respectively [20,52,53] (Figure 1). As a matter of fact, Ito et al. observed that, anti-OX40L or anti-IL-4 monoclonal antibody strongly inhibited the production of IL-4, IL-5 and IL-13 [53,54]. Respectively, there is clear evidence which support that TSLP, even in the absence of IL-4, could directly promote Th2 differentiation and type 2 cytokine from naive T cells in vitro [55].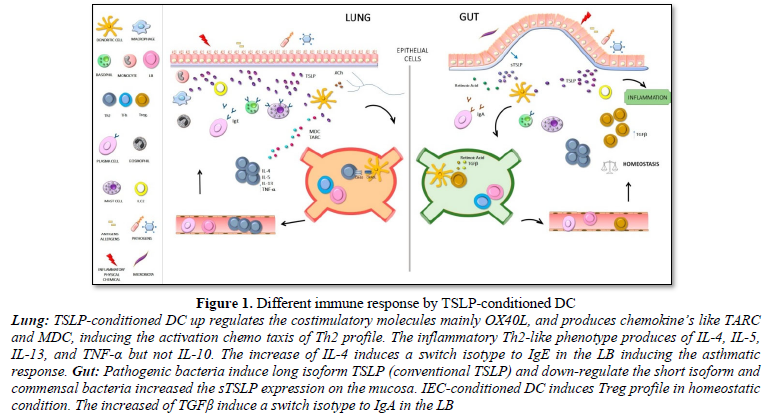
Taking into account all the information mentioned before, the Th2 profile is induced and the recruitment of Th2 cells favors them to migrate towards inflammatory sites and reflects the disease activity in pathologies like dermatitis, asthma, allergic responses, [32,51].
Interestingly, our group observed that DC cultured with Acetylcholine (ACh); the most important parasympathetic neurotransmitter in the airways [56]; in presence of TSLP showed higher levels of OX40L expression than cells cultured with individual stimuli. A similar effect was observed with the expression of maturation markers and the TNF-α and IL-8 cytokine production. Moreover, when DC were cultured with both TSLP and ACh, a higher stimulation of IL-4, IL-5, and IL13 production was observed, all of that, suggesting that a neurotransmitter like ACh combined with TSLP-stimulated DC could enhance the Th2 profile polarization facilitating, in consequence, the development of asthma [57].
Homeostatic function
At the beginning of this review, we describe several factors capable of inducing TSLP secretion by epithelial cells, especially during the inflammatory response, but this is also critical for the generation and maintenance of the homeostatic microenvironment, in which DC again is one of the main protagonists [41,58,59].
FUNCTIONAL ROLE OF TSLP IN THE GUT
For a few years, attempts have been made to deepen its action on the intestinal mucosa and the pathologies associated with its immune dysregulation. The gastrointestinal tract is the largest surface of the body and the most exposed to potentially pathogenic microorganisms. It has a role of allowing the uptake of micronutrients and preventing the entry of microorganisms while maintaining homeostasis [60]. Multiple mechanisms of both innate and adaptive immunity participate in maintaining mucosal homeostasis [61]. Indeed, it is the epithelial cells that play an essential role. This physical barrier that separates the lumen from immune cells includes tight junctions, produces antimicrobial peptides and mucins that prevent the adherence and subsequent colonization of microorganisms but it also secretes constitutive factors and cytokines such as TGFβ and IL-10 that maintain the mucosa tolerance not only to microbial challenge but also to dietary antigens [62,63]. In fact, the breakdown of this barrier leads to the development of inflammatory diseases as allergies, diabetes and inflammatory bowel diseases (IBD). In recent years, intestinal epithelial cells (IEC) have acquired central importance in sensing the environment and instructing dendritic cells in intimate contact to act accordingly [64,65]. Thus, CD103+ dendritic cells in the presence of a non-activated line of human IEC acquire an anti-inflammatory phenotype. The gastrointestinal tract expresses TSLP constitutively, with low levels in the small intestine and higher in the colon [24]. The TSLP was established as one of the main factors secreted by the IEC when sensing the flora; modulate the basal levels of this hematopoietic factor for instructing DC towards a non-inflammatory profile [58]. EIC-conditioned mucosal DC increase the expression of the OX40L molecule inducing a Th2 profiles while decreasing the expression of IL-12/23 p40 subunit, effects mediated by TSLP and limiting Th1/Th17 polarization reducing the production of IFN-g and IL- 17 [48,66,67]. In the same way, conditioned DC acquires a non-inflammatory phenotype activating the differentiation of Foxp3 Treg cells suppressing immune response and inducing tolerance [68]. In vitro assays with naive CD4+ CD25- cells showed that TSLP per se is not capable of inducing CD4+CD25+ Foxp3+ Treg cells [6, 69]. This only occurs when TSLP previously interacted with DC of mesenteric lymph node or lamina propria. The regulatory phenotype acquisition is not induced when conditioned DC are co-cultured with peripheral naive lymphocytes [70]. The knockout (KO) of the RTSLP in DC was shown to prevent the induction of Foxp3 Treg lymphocytes in a murine model [71]. Facts which reinforce the essential TSLP role in the homeostasis control of the gastrointestinal tract.
Variations in TSLP levels in the intestinal mucosa are essential to define the degree of activation of the DC and the bias of the concomitant effector response. Low concentrations maintain low IL-2 secretion and favor non-inflammatory Th2 polarization. The bacterial ligands of toll like receptors (TLR) such as lipopolysaccharide (LPS), peptidoglycan and flagellin when interacting with IEC increase TSLP levels in the mucosa, mediated effect via the activation of the NFk-β pathway [72-74]. Also, viral components of rotavirus and human immunodeficiency virus (HIV) increase TSLP levels when sensed by the IEC [75]. Minimal variations in these basal concentrations instruct DC into an inflammatory profile capable of producing IL-12 and induce inflammatory Th1 response profiles [24].
FUNCTIONAL ROLE OF TSLP IN THE THYMUS
The thymic stroma is mainly made up of a heterogeneous population of epithelial cells, thymic epithelial cells, (TEC) present in both the cortex and the medulla, which are called cortical thymic epithelium (TECc) and medullary (TECm) cells, respectively. It is known that TECc are involved in the process of positive selection, while TECm and thymic DC are involved in the process of negative selection [76] . As regards, TSLP, it is expressed in Hassall’s corpuscle where also in the medulla localized activated DC, and CD4+ CD25+ Treg. Due to its expression by Hassal´s corpuscles in the thymus, TSLP has homeostatic activities like regulation on the capacity of DC and plasmacytoid DC to drive development of Treg [58,77,78]. Interestingly, the number of Foxp3+ Treg in the tumor microenvironment correlated with the increase expression of TSLP protein in some tumor, like lung cancer [79].
FUNCTIONAL ROLE OF TSLP IN TROPHOBLASTIC AND PREGNANCY
Physiologically, pregnancy can be considered a successful embryo allograft. Guo et al. [42] described that human trophoblast, cells secreted soluble TSLP in maternal-fetal interface of early placentas. They described that the functional RTSLP is highly expressed in human decidual CD1c DC (dDC) and, besides, TSLP or supernatants from human trophoblasts culture specifically stimulate dDC to highly produce interleukin-10 and Th2-attracting chemokine TARC/CCL-17. The TSLP-conditioned dDC prepare decidual CD4+ T cells for Th2 cell differentiation, involved in maternal-fetal immunotolerance. Moreover, the combination of hormones like progesterone or estradiol at physiological levels in early human pregnancy also induces TSLP mRNA and protein expression [43,80], too deep in this theme, Lin et al. [80,81] discover that, in a murine model, TSLP-conditioned DC can boost the production of TARC/CCL17, which can afterwards, attract Th2-type cells to immigrate into the uterus. In addition, Du et al. [82] proposed a crosstalk model between embryo trophoblasts and decidual leukocyte subsets of the maternal–fetal interface in human first-trimester pregnancy, they confirmed, trophoblast-derived TSLP actives the DC to induce CD4+ CD25+ FOXP3+ T cells profile in early pregnancy via TGF-β1. In summary the TSLP is critical for a successful pregnancy, mainly in the beginning of the maternal-fetal interface.
TSLP and a new paradigm
The difference between the activation of DC with the induction of homeostatic in certain tissues and the hallmark of exacerbated Th2 profile on the microenvironment where the DC were found, is an issue that generates the beginning of the paradigm of the function of TSLP. Recently, different groups have shown that exists in humans a novel isoform, that is to say, a shorter isoform of TSLP who is constitutively expressed in a variety of tissues, including bronchial and colonic epithelial cell, keratinocytes and lung fibroblasts [42,83]. Furthermore, short TSLP isoform (sTSLP) is involved in homeostatic functions, whereas the long TSLP isoform (lTSLP, conventional isoform) is expressed constantly at a very low level and up regulated during inflammation in different tissues [24]. Fornasa et al. [84] described the two coding transcripts code for the lTSLP of 159 amino acids and for sTSLP which has the last 63 residues of lTSLP and is identical to its C-terminal portion. They describe that sTSLP is the homeostatic isoform of TSLP present under steady-state conditions in the gut and skin. They described whether sTSLP had anti-inflammatory properties on DC. However, only lTSLP significantly up regulated TARC/CCL17 and MDC/CCL22 expression and the secretion of TNF alfa, but was not affected, in none of the 3 cytokines, by the presence of sTSLP. Finally, Tsilingiri et al. [24] reported that the 2 isoforms were not the result of alternative splicing of the same transcript; they are controlled by two different promoter regions.
Interestingly, a dual tissue-dependent role is assigned to TSLP. In general, its role is inflammatory in the skin and lung and anti-inflammatory in the intestine and thymus. In the intestine they contribute to maintain homeostasis through the induction of regulatory response profiles. In part, the immunomodulatory effect was attributed to the sTSLP whose transcript is most expressed in the epithelial barrier. The lack of animal models, which do not express the short isoform and the fact that TSLPR-IL7Rα heterodimeric receptor, is for the long form makes a functional study difficult. However, the fact that IEC in contact with pathogenic bacteria up- regulates the long isoform and down-regulates the short one while the contrary is observed with commensal bacteria supports the theory of homeostatic action of the short form on the mucosa [24,85,86].
TSLP IN PATHOLOGIES
Asthma
Asthma is a chronic inflammatory disease of the conducting airways that involving a series of events with the participation of epithelial cells and the activation of immune cell effector mechanisms It is known that this series of events involving the airways is associated with the development of a Th2 profile, airway inflammation, bronchial hyper reactivity, the excessive production of mucous secretion and the structural remodeling of the airway. Th2 lymphocytes with the consequent production of IL-4, IL-5 and IL-13 cytokines; lead to chronic inflammation characterized by infiltration of the mucosa of eosinophil’s, mast cells, and Th2 lymphocytes [46,87,88]. As the DC are the orchestral conductors of the immune response, imposing a specific Th lymphocyte profile, their ability to sense the surrounding microenvironment is of utmost importance for the initiation of allergic processes.
As described previously the TSLP secreted by epithelial cell is the cytokine responsible for conditioning DC to a Th2 inflammatory profile that produce the classical Th2 cytokines IL-4, IL- 5, and IL-13, and a high concentration of TNF-α promoting development to asthma pathogenesis [20,32]. Furthermore, an experiment made in TSLPR KO mice failed to develop an inflammatory lung response, underlining the importance for this cytokine in the development in allergic response [11]. The TSLP overexpressed in airway epithelia lung biopsies of asthmatic patients [89,90] and in asthmatic mice, [2,91] which is associated to the pathogenesis of airway disease, correlated with the severity of asthma. Moreover, a polymorphism in the TSLP locus was associated with an increased risk or more susceptibility in development of asthma [92,93].
Studies carried out in serum samples of 65 pediatric patients, newly diagnosed for allergic asthma, showed an increased production of the TSLP that correlated negatively with asthma control test samples and Treg cells [94]. Different groups proposed the TSLP as a biomarker for inflammation asthma patients and also as a biomarker of severe asthma [94,95]. As described previously the TSLP may have dual immunoregulatory roles. Dong et al. [96] found that house dust mite (HDM) and lTSLP impaired barrier function and the treatment with sTSLP and 1,25D3 prevented HDM-induced airway epithelial barrier disruption. Moreover, sTSLP and 1,25D3 treatment ameliorated HDM-induced asthma in mice.
The relevance of TSLP towards the induction of a Th2 profile and the development of the asthmatic process is not limited to its effect on DC, other cell types favor this profile such as mast cells, basophils [1,32] and Innate lymphoid cells 2 (ILC2), in the last one mainly his survival [97]. ILCs are a recently identified family of heterogeneous immune cells that can be divided into three groups based on their differential developmental requirements and expression of effector cytokines. The ILC2s produce the type 2 cytokines interleukin-5 (IL-5) and IL-13 and promote type 2 inflammation in the lung and intestine. Kabata et al. [98] suggest that the ILC2 priming by TSLP may play a critical role in the resistance to steroid in allergic airway inflammation.
TSLP and viral infection
Respiratory virus infections, such as respiratory syncytial virus (RSV) and rhinovirus infections have been associated, in children and adults, with the development of persistent or exacerbations asthma. Indeed, rhinovirus infection in the first 3 years of life is associated with increase in risk for asthma [99,100]. Viral infection may development of the Th2 immune response, be part of leading to reduced IFN-γ and IL-12, and inefficient antiviral immunity asthmatic individuals [101], by activation of different TLR [102].
Tanaka et al. [103] evaluated how the relationship between TSLP and TLR3 ligand stimulation influences DC activation. They suggested that through DC activation, human TSLP and TLR3 ligands promote differentiation of Th17 cells with the central memory T cell phenotype under Th2- polarizing conditions. This result is relevant to patients with severe asthmatic disease who have a neutrophil infiltrate and inflammation, probably induced by the Th17 profile.
Lee et al. [104] reported RIG-I as a novel pathway that leads to TSLP expression after respiratory virus infection of airway epithelial cell, confirming that airway epithelial cells from asthmatic children produce significantly greater levels of TSLP after RSV infection than cells from healthy children. On the other hand, they confirm that RSV-induced TSLP expression was found to be critical for the development of immunopathology, in a murine model.
Conversely to the previously mentioned works, there would seem not to be a beneficial role of TSLP in antiviral immunity, in fact, studies realized with TSLPR-deficient mice, show that TSLP was required for the expansion and activation of virus-specific effector CD8 +T cells in the lung, but not in the lymph node. The mechanism involved TSLPR signaling on newly recruited CD11b+ inflammatory DC [105]. TSLP may be the connections between virus infection and persistent or exacerbations asthma.
Atopic dermatitis
Atopic dermatitis (AD) is a common chronic skin disorder, with relapsing eczematous skin inflammation often accompanying severe pruritus [106].
Soumelis et al. [50] determined in the 2002, the expression of TSLP protein in of skin lesions, atopic dermatitis, nickel-induced contact dermatitis and cutaneous lupus erythematous samples. High expression of TSLP was found in the keratinocytes of acute and chronic atopic dermatitis, a clear Th2 profile of allergic disease. This group determined also that the expression of TSLP was associated with the activation of Langerhans cells. Murine models confirmed that DC migrate to lymph nodes and activate to Th2 profile [107].
Moreover, like in asthma patients, important concentration of the TSLP detected in serum of patients both children and adults with AD [108-110]. Polymorphisms in the TSLP gene are associated with an increased risk of development and progression of AD. In this pathology the polymorphisms can involve both TSLP and its RTSLP or RIL-7 [110,111].
Perinatal supplementation with probiotics has been shown to reduce the incidence of AD in infancy [112]; as one of the cytokines found in breast milk is TSLP [113] it was postulated that the mechanism that reduces the AD involved this cytokine, but neither TSLP nor TGFβ would seem to be involved [114].
One of the most interesting research of the last years, described a directly communication between epithelial cells to cutaneous sensory neurons via TSLP to promote itch. TSLP acts directly on a subset of TRPA1-positive sensory neurons to trigger robust itch behaviors, giving other clear evidence of the influence of the nervous system on allergic pathologies [30].
Both Basophils [115] and ILC2 have a significant relevance in TSLP activation in AD. In fact, a population of skin resident ILC2s present in healthy human skin was identified by Kim et al. [116] besides this is enriched of these cells in lesioned human skin from AD patients. ILC2 is mainly regulated by IL-25 and IL-33 in gut and lung, but Kim et al. [116] described that the ILC2 in skin and skin-draining lymph nodes responds critically to TSLP. Finally, TSLP interacts directly with skin-homing Th2 cells in AD patients which have enhanced TSLPR expression [117].
Fornasa et al. [84] found an up regulation of the lTSLP isoform in lesioned as opposed to nonlesional biopsy specimens but they found that sTSLP was significantly down regulated in lesioned biopsy, indicating an imbalance of the 2 isoforms in patients with AD because they showed down regulation of sTSLP and up regulation of lTSLP.
GUT PATHOLOGIES
Epithelial cells of the mucosa and dysbiosis of the micro biota are pillars in the development of inflammatory bowel diseases. IBD refers to two entities defined as ulcerative colitis (UC) and Crohn disease (CD) [62,118]. These diseases have a high prevalence of 396 per 100,000 individuals, values that increase year by year. Because of the symptoms with which they occur such as diarrhea, abdominal pain, and weight loss, they are considered disabling diseases. Due to the homeostatic function of TSLP in the intestine plus the fact that it is produced in a constitutive homeostatic way, it is assumed that alterations in these levels are associated with pathology. The expression of TSLP was demonstrated in the colon lesions of patients with UC whose effector mechanism is the induction of Th2 lymphocytes. In contrast, in colon biopsies of patients with Crohn’s disease, characterized by Th1/Th17 response profiles, down regulation of the TSLP gene was described [70], strikingly when their CDs when stimulated by bacterial ligands they secrete IL-12, which is consistent with the inability in these patients to induce tolerogenic or non-inflammatory DC [85,119]. Likewise, in vitro tests with DC derived from human monocytes are only observed at low concentrations of TSLP, not when the concentrations are high. Indicating the existence of a concentration window outside of which the inflammatory response is triggered.
Gene association studies found a correlation of TSLP with genes associated with the development of IBD. The most notable is that the CCR5 and CLCX10 chemokine receptors that govern the migration of T lymphocytes to the epithelium are up regulated by TSLP, which is essential in the development of necessary Th2 letters associated with UC [120,121]. Another Th2 chemo attractant is CCL11, which is increased in biopsies of UC patients and CCR2 that allows homing of intraepithelial lymphocytes that express the αEβ7 molecule up regulated by TSLP in UC [70]. In contrast, the decrease of CCL11 in CD was not associated with TSLP [122]. IL-4 and Il-13 cytokines are increased by TSLP in the colon of UC patients. It was demonstrated that IL-13 induces an increase of the permeability in the IECs mediated by the activation of cellular apoptosis and the decrease of the ocludin 2 leading to the damage associated with this pathology [123, 124]. Genes are also up regulated in DCs that have to do with the induction of the Th2, CCL24/eotaxin profile that induce eosinophil recruitment in UC patients [125].
It should be noted that an association was also found between the levels of TSLP and genes associated with the junctions of epithelial cells. In this sense, a decrease in Zo-1 and ocludin, a protein that is part of tight junctions in UC patients, was found. Disruption of barrier permeability is known to be one of the first mechanisms in inducing inflammatory response associated with damage to the mucosa and pathology, on the contrary, the CLN1 gene is increased, which produces ocludin-1, a mechanism associated with compensating for damage [70,126,127].
An attempt has been made to define the association of isoforms with each pathology. The most relevant results show that in the biopsies of patients with CD the short isoform is down- regulated; while there would be no alteration in the long isoform [127], the opposite effect occurs in biopsies of patients with UC. Tsilingiri et al. [84] using specific-isoform antibodies demonstrated a down-regulation of short isoform in the biopsies of untreated celiac patients. These results encourage future therapies to restore the homeostatic levels of this isoform [128]. The results are generally consistent in that the long isoform would be responsible for the induction of disease-associated damage. All this allows us to think that blocking TSLPR-TSLP signaling would be encouraging in the development of therapies that improve the quality of life of patients with IBD interfering with the activation of the inflammatory response through the restoration of homeostatic conditions.
Conclusion and therapeutic target
In conclusion, the TSLP is a key modulator of the responses through its impact mainly on DC. Due to the relevance of TSLP in the pathophysiology of diseases such as asthma and atopic dermatitis, the blocking of this cytokine has been the target of important research, leading to development of a numerous of clinical trials with very promising results to the treatment of this pathologies [1,21,110,129].
- Roan F, Obata-Ninomiya K, Ziegler SF (2019) Epithelial cell-derived cytokines: More than just signaling the alarm. J Clin Invest 129: 1441-1451.
- Lambrecht BN, Hammad H (2012) The airway epithelium in asthma. Nat Med 18: 684-92.
- Varricchi G, Marone G, Pecoraro A, Marone G, Criscuolo G, et al. (2018) Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front Immunol 9: 1595.
- Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, et al. (2001) Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol 167: 336-343.
- Guermonprez P, Valladeau J, Zitvogel L, Théry C, Amigorena S (2002) Antigen presentation and T cell stimulation by dendritic cells. Ann Rev Immunol 20: 621-667.
- Liu YJ, Soumelis V, Watanabe N, Ito T, Wang YS, et al. (2007) TSLP: An Epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Ann Rev Immunol 25: 193-219.
- Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, et al. (1994) A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol 22: 321-328.
- Sims JE, Williams DE, Morrissey PJ, Garka K (2000) Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med 192: 671-680.
- Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M (2001) Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia 15: 1286-1292.
- Ray RJ, Furlonger C, Williams DE, Paige CJ (1996) Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol 26: 10-16.
- Al-Shami A, Spolski R, Kelly J, Keane-Myers A (2005) A role for TSLP in the development of inflammation in an asthma model. J Exp Med 202: 829-839.
- Sebastián K, Borowski A, Kuepper M, Friedrich K (2008) Signal transduction around thymic stromal lymphopoietin (TSLP) in atopic asthma. Cell Commun Signal 6: 5.
- Park LS, Martin U, Garka k, Di Santo JP, Muller W, et al. (2000) Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med 192: 659-670.
- Pandey A, Ozaki KN, Baumann H, Levin SD (2000) Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol 1: 59-64.
- Calven J, Yudina Y, Hallgren O, Westergren-Thorsson G (2012) Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: Role of endosomal TLR3 and cytosolic RIG-I-like helicases. J Innate Immun 4: 86-99.
- Cultrone A, de Wouters T, Lakhdari O, Kelly D, Mulder I, et al. (2013) The NF-kB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur J Immunol 43: 1053-1062.
- Kashyap M, Rochman Y, Sploski R, Samsel L, Leonard WJ (2011) Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol 187: 1207-1211.
- Allakhverdi Z, Comeau MR, Jessup HK, Delespesse G (2009) Thymic stromal lymphopoietin as a mediator of crosstalk between bronchial smooth muscles and mast cells. J Allerg Clin Immunol 123: 9 58-960.
- Tonozuka Y, Fujio K, Sugiyama T, Nosaka T, Hirai M, et al. (2001) Molecular cloning of a human novel type I cytokine receptor related to delta1/TSLPR. Cytogenet Cell Genet 93: 23-25.
- Liu YJ (2009) TSLP in epithelial cell and dendritic cell cross talk. Adv Immunol 101: 1-25.
- Cianferoni A, Spergel J (2014) The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev Clin Immunol 10: 1463-1474.
- Doherty TA, Baum R, Newbury Ro, Kurten RC, Broide DH (2015) Group 2 innate lymphocytes (ILC2) are enriched in active eosinophilic esophagitis. J Allergy Clin Immunol 136: 792-794.
- Demehri S, Liu Z, Lee J, Lin MH, Crosby SD (2008) Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol 6: 123.
- Tsilingiri K, Fornasa G, Rescigno M (2017) Thymic Stromal Lymphopoietin: To cut a long story short. Cell Mol Gastroenterol Hepatol 3: 174-182.
- Pattarini L, Trichot C, Bogiatzi, Grandclaudon (2015) TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med 214: 1529-1546.
- Wong CK, Hu S, Cheung PFY, Lam CWK (2010) Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol 43: 305-315.
- Schwartz C, Eberle JU, Voehringer D (2016) Basophils in inflammation. Eur J Pharmacol 778: 90-95.
- Allakhverdi Z, Comeau MR, Jessup HK, Yoon BRP, Brewer A, et al. (2007) Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med 204: 253-258.
- Rochman Y, Leonard WJ (2008) The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol 181: 7699-7705.
- Wilson SR, The L, Batia LM, Pellegrino M, Estadian DM, et al. (2013) The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155: 285-295.
- Briot A, Deraison C, Lacroix M, Bonnart C (2009) Kallikrein 5 induces atopic dermatitis-like lesions through PAR2-mediated thymic stromal lymphopoietin expression in Netherton syndrome. J Exp Med 206: 1135-1147.
- Corren J, Ziegler SF (2019) TSLP: from allergy to cancer. Nat Immunol 20: 1603- 1609.
- Volpe E, Pattarani L, Martinez-Cingolani C, Meller S, Donnadieu MH (2014) Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J Allergy Clin Immunol 134: 373-3781.
- Moret FM, Hack CE, van der Wurff-Jacobs KMG, Radstake TRD, Lafeber FPJG, et al. (2014) Thymic stromal lymphopoietin, a novel proinflammatory mediator in rheumatoid arthritis that potently activates CD1c+ myeloid dendritic cells to attract and stimulate T cells. RA 66: 1176-1184.
- Kuan EL, Ziegler SF (2018) A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol 19: 366-374.
- Astrakhan A, Nguyen T, Omari M, Becker-Herman S (2007) Local increase in thymic stromal lymphopoietin induces systemic alterations in B cell development. Nat Immunol 8: 522-531.
- Takahashi N, Sugaya M, Suga H, Oka T, Kawaguchi M, et al. (2016) Thymic Stromal Chemokine TSLP Acts through Th2 Cytokine Production to Induce Cutaneous T-cell Lymphoma. Cancer Res 76: 6241-6252.
- Burkard-Mandel L, Neill RO, Colligan S, Seshadri M, Abrams SI (2018) Tumor-derived thymic stromal lymphopoietin enhances lung metastasis through an alveolar macrophage-dependent mechanism. Oncoimmunology 7: e1419115.
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, et al. (2011) Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 208: 469-478.
- Watanabe N, Lee HK, Ito T, Wang YH (2005) Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436: 1181-1185.
- Coombes JL, Powrie F (2008) Dendritic cells in intestinal immune regulation. Nat Rev Immunol 8: 435-446.
- Guo PF, Du MR, Wu HX, Lin Y, Jin LP, et al. (2010) Thymic stromal lymphopoietin from trophoblasts induces dendritic cell- mediated regulatory TH2 bias in the decidua during early gestation in humans. Blood 116: 2061-2969.
- Wu HX, Guo PF, Jin LP, Liang SS, Li DJ (2010) Functional regulation of thymic stromal lymphopoietin on proliferation and invasion of trophoblasts in human first-trimester pregnancy. Hum Reprod 25: 1146-1152.
- Steinman RM, Hawiger D, Nussenzweig MC (2003) Tolerogenic dendritic cells. Annu Rev Immunol 21: 685-711.
- Ardavin C, Amigorena, e Sousa CR (2004) Dendritic cells: Immunobiology and cancer immunotherapy. Immunity 20: 17-23.
- Sabatte J, Maggini J, Nahmod K, Amaral MM, Martinez D, et al. (2007) Interplay of pathogens, cytokines and other stress signals in the regulation of dendritic cell function. Cytokine Growth Factor Rev 18: 5-17.
- Ebner S, Nguyen VA, Forstner M, Wang YH, Wolfram D, et al. (2007) Thymic stromal lymphopoietin converts human epidermal Langerhans cells into antigen-presenting cells that induce proallergic T cells. J Allergy Clin Immunol 119: 982-990.
- Wang J, Xing F (2008) Human TSLP-educated DCs. Cell Mol Immunol 5: 99-106.
- Ito T, Wang YH, Duramad O, Hori T (2005) TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med 202: 1213-1223.
- Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, et al. (2002) Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol 3: 673-680.
- Ziegler SF (2012) Thymic stromal lymphopoietin and allergic disease. J Allergy Clin Immunol 130: 845-852.
- Croft M (2010) Control of immunity by the TNFR-related molecule OX40 (CD134). Annu Rev Immunol 28: 57-78.
- Watanabe N, Hanabuchi S, Marloie- Provost MA, Antonenko S, Liu YJ, et al. (2005) Human TSLP promotes CD40 ligand-induced IL-12 production by myeloid dendritic cells but maintains their Th2 priming potential. Blood 105: 4749-4751.
- Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, et al. (2007) In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest 117(12): 3868-3878.
- Rochman Y, Dienger-Stambaugh K, Richgels PK, Lewkowich IP, Kartashov AV, et al. (2018) TSLP signaling in CD4 (+) T cells programs a pathogenic T helper 2 cell state. Sci Signal 11.
- Kistemaker LE, Gosens R (2014) Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci 36(3): 164-171.
- Gori S, Vermeulen M, Lenicov FR, Jancic C, et al. (2017) Acetylcholine polarizes dendritic cells toward a Th2-promoting profile. Allergy 72: 221-231.
- Hanabuchi S, Watanabe N, Liu YJ (2012) TSLP and immune homeostasis. Allergol Int 61: 19-25.
- Watanabe N, Hanabuchi S, Soumelis V, Ho S, Yuan W, et al. (2004) Human thymic stromal lymphopoietin promotes dendritic cell- mediated CD4+ T cell homeostatic expansion. Nat Immunol 5: 426-434.
- Andrews C, McLean MH, Durum SK (2018) Cytokine tuning of intestinal epithelial function. Front Immunol 9: 1270.
- Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474: 1823-1836.
- Okumura R, Takeda K (2017) Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med 49: e338.
- Gunther J, Seyfert HM (2018) The first line of defence: insights into mechanisms and relevance of phagocytosis in epithelial cells. Semin Immunopathol 40: 555-565.
- Stagg AJ (2018) Intestinal dendritic cells in health and gut inflammation. Front Immunol 9: 2883.
- Lin L, Zhang J (2017) Role of intestinal microbiota and metabolites on gut homeostasis and human diseases. BMC Immunol 18: 2.
- Ziegler SF, Artis D (2010) Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol 11: 289-293.
- Fuss IJ (2008) Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm Bowel Dis 14: 110-112.
- Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, et al. (2009) Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut 58: 1481-1489.
- Aubry C, Michon C, Chain F, Chvatchko Y, Goffin L, et al. (2015) Protective effect of TSLP delivered at the gut mucosa level by recombinant lactic acid bacteria in DSS-induced colitis mouse model. Microb Cell Fact 14: 176.
- Park JH, Jeong DY, Peyrin- Biroulet L, Eisenhut M (2017) Insight into the role of TSLP in inflammatory bowel diseases. Autoimmun Rev 16: 55-63.
- Goodman WA, Pizarro TT (2013) Regulatory cell populations in the intestinal mucosa. Curr Opin Gastroenterol 29: 614-620.
- Mosconi I, Geuking MB, Ziass M, Massacand JC, Aschwanden C, et al. (2013) Intestinal bacteria induce TSLP to promote mutualistic T-cell responses. Mucosal Immunol 6: 1157-1167.
- Brand S (2009) Crohn's disease: Th1, Th17 or both? The change of a paradigm: new immunological and genetic insights implicate Th17 cells in the pathogenesis of Crohn's disease. Gut 58: 1152-1167.
- Soumelis V, Liu YJ (2003) Human thymic stromal lymphopoietin: a novel epithelial cell- derived cytokine and a potential key player in the induction of allergic inflammation. Springer Semin Immunopathol 25: 325-333.
- Li M, Zhang J, Wu Y, Li J (2011) The regulation of thymic stromal lymphopoietin in gut immune homeostasis. Dig Dis Sci 56: 2215-2220.
- Kumar BV, Connors TJ, Farber Dl (2018) Human T cell development, localization, and function throughout Life. Immunity 48: 202-213.
- Lee HM, Hsieh CS (2009) Rare development of Foxp3+ thymocytes in the CD4+CD8+ subset. J Immunol 183: 2261-2266.
- Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, et al. (2010) Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol 184: 2999-3007.
- Li H, Zhao H, Yu J, Su Y, Cao S, et al. (2011) Increased prevalence of regulatory T cells in the lung cancer microenvironment: a role of thymic stromal lymphopoietin. Cancer Immunol Immunother 60: 1587-1596.
- Zhang Y, Jin LP (2016) Effects of TSLP on obstetrical and gynecological diseases. Am J Reprod Immunol 77.
- Lin Y, Wang W, Jin H, Zhong Y, Di J, et al. (2009) Comparison of murine thymic stromal lymphopoietin- and polyinosinic polycytidylic acid-mediated placental dendritic cell activation. J Reprod Immunol 79: 119-128.
- Du MR, Guo PF, Piao HL, Wang SC, Sun C, et al. (2014) Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol 192: 1502-1511.
- Harada, M, Hirota T, Jodo AI, Doi S, Kameda M, et al. (2009) Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol 40: 368-374.
- Fornasa G, Tsilingiri K, Caprioli F, Longhi R, Penna G, et al. (2015) Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol 136: 413- 422.
- Rimoldi M, Chieppa M, Salucci V, Avogadri F, Sonzogni A, et al. (2015) Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol 16: 326.
- Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, et al. (2009) TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 206: 655-667.
- Wills-Karp M (1999) Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol 17: 255-281.
- Hammad H, Lambrecht BN (2006) Recent progress in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation. J Allergy Clin Immunol 118: 331-336.
- Ying S, Connor BO, Ratoff J, Meng Q, Mallett K, et al. (2005) Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol 174: 8183-8190.
- Shikotra A, Choy DF, Ohri CM, Heaney LG, Arron JR, et al. (2012) Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol 129: 104-111.
- Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, et al. (2005) Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol 6: 1047-1053.
- Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux C, et al. (2011) Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet 43: 887-892.
- Hirota T, Takahasi A, Kubo M, Tsunoda T, Tomita K, et al. (2011) Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet 43: 893-896.
- Chauhan A, Singh M, Agarwal A, Paul N (2015) Correlation of TSLP, IL-33, and CD4 + CD25 + FOXP3 + T regulatory (Treg) in pediatric asthma. J Asthma 52: 868-872.
- Li Y, Wang W, Zhe LV, Li Y, Chen Y, et al. (2018) Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J Immunol 200: 2253-2262.
- Dong H, Hu Y, Liu L, Zou M, Huang C, et al. (2016) Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep 6: 39559.
- Barnes PJ (2018) Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 18: 454-466.
- Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, et al. (2013) Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun 4: 2675.
- Lemanske RF, Jackson DJ, Gangnon RE, DaSilva DF, Tisler CJ, et al. (2005) Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol 116: 571-577.
- Perez GF, Pancham K, Huseni S, Preciado D, Freishtat RJ, et al. (2014) Rhinovirus infection in young children is associated with elevated airway TSLP levels. Eur Respir J 44: 1075-1078.
- Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, et al. (1999) Rhinovirus-16 colds in healthy and in asthmatic subjects: similar changes in upper and lower airways. Am J Respir Crit Care Med 160: 100-108.
- Esnault S, Rosenthal LA, Wang DS, Malter JS (2008) Thymic stromal lymphopoietin (TSLP) as a bridge between infection and atopy. Int J Clin Exp Pathol 1: 325-330.
- Tanaka J, Watanabe N, Kido M, Saga K, Akamatsu T, et al. (2009) Human TSLP and TLR3 ligands promote differentiation of Th17 cells with a central memory phenotype under Th2-polarizing conditions. Clin Exp Allergy 39: 89-100.
- Lee HC, Headley MB, Loo YM, Debley JS, Lukacs NW, et al. (2012) Thymic stromal lymphopoietin is induced by respiratory syncytial virus- infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol 130: 1187-1196.
- Yadava K, Sichelstiel A, Luescher IF, Nicod LP, Harris LN, et al. (2013) TSLP promotes influenza-specific CD8+ T-cell responses by augmenting local inflammatory dendritic cell function. Mucosal Immunol 6: 83-92.
- Bieber T (2008) Atopic dermatitis. N Engl J Med 358: 1483-1494.
- Oyoshi MK, Larson RP, Ziegler SF, Geha RS (2010) Mechanical injury polarizes skin dendritic cells to elicit a T (H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol 126: 976-984.
- Nygaard U, Hvid M, Johasen C, Buchner M, Deleuran M, et al. (2016) TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol 30: 1930-1938.
- Lee EB, Kim KW, Hong JY, Jee HM, Sohn MH, et al. (2010) Increased serum thymic stromal lymphopoietin in children with atopic dermatitis. Pediatr Allergy Immunol 21: 457-460.
- Nakajima S, Kabata H, Kabashima K, Asano K (2020) Anti-TSLP antibodies: Targeting a master regulator of type 2 immune responses. Allergol Int 69: 197-203.
- Gao PS, Rafaels NM, Mu D, Beck LA, Leung DYM, et al. (2010) Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol 125: 1403-1407.
- Pelucchi C, Lilliane C, Federica T, Carlotta G, Lorenzo M, et al. (2012) Probiotics supplementation during pregnancy or infancy for the prevention of atopic dermatitis: a meta-analysis. Epidemiology 23: 402-414.
- Macfarlane TV, Seager AL, Moller M, Morgan G, Thorton CA, et al. (2010) Thymic stromal lymphopoietin is present in human breast milk. Pediatr Allergy Immunol 21: 454-456.
- Simpson MR, Bjerkenes Ro AD, Grimstad O, Johnsen R, Storro O, et al. (2016) Atopic dermatitis prevention in children following maternal probiotic supplementation does not appear to be mediated by breast milk TSLP or TGF-beta. Clin Transl Allergy 6: 27.
- Nakashima C, Otsuka A, Kabashima K (2018) Recent advancement in the mechanism of basophil activation. J Dermatol Sci 91: 3-8.
- Kim BS, Siracusa MC, Saenz SA, Noti M, Monticelli LA, et al. (2013) TSLP elicits IL-33-independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med 5: 170ra16.
- Tatsuno K, Fujiyama T, Yamaguchi H, Waki M, Tokura Y (2015) TSLP Directly Interacts with Skin-Homing Th2 Cells Highly Expressing its Receptor to Enhance IL-4 Production in Atopic Dermatitis. J Invest Dermatol 135: 3017-3024.
- Weiss GA, Hennet T (2017) Mechanisms and consequences of intestinal dysbiosis. Cell Mol Life Sci 74: 2959-2977.
- Spadoni I, Ileiv ID, Rossi G, Rescigno M (2012) Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol 5: 184-193.
- Thomas S, Baumgart DC (2012) Targeting leukocyte migration and adhesion in Crohn's disease and ulcerative colitis. Inflammopharmacology 20: 1-18.
- Herfarth H, Pollok-Kopp B, Göke M, Press A, Oppermann M (2001) Polymorphism of CC chemokine receptors CCR2 and CCR5 in Crohn's disease. Immunol Lett 77: 113-117.
- Manousou P, Kolios C, Valatas Y, Drygiannakis I, Bourikas L, et al. (2010) Increased expression of chemokine receptor CCR3 and its ligands in ulcerative colitis: The role of colonic epithelial cells in in vitro studies. Clin Exp Immunol 162: 337-347.
- Rosen MJ, Frey MR, Washington KM, Chaturvedi R, Kuhnhein LA, et al. (2011) STAT6 activation in ulcerative colitis: a new target for prevention of IL- 13-induced colon epithelial cell dysfunction. Inflamm Bowel Dis 17: 2224-2234.
- Schwarzmaier D, Foell D, Weinhage T, Varga G, D et al. (2013) Peripheral monocyte functions and activation in patients with quiescent Crohn's disease. PLoS One 8: e62761.
- Lampinen M, Carlson M, Waddell A, Ahrens R, Hogan S (2013) CD14+CD33+ myeloid cell-CCL11-eosinophil signature in ulcerative colitis. J Leukoc Biol 94: 1061-1070.
- Poritz LS, Garver KI, Green C, Fitzpatrick L, Ruggiero F et al. (2007) Loss of the tight junction protein ZO-1 in dextran sulfate sodium induced colitis. J Surg Res 140: 12-19.
- Neurath MF (2014) Cytokines in inflammatory bowel disease. Nat Rev Immunol 14: 329-342.
- Biancheri P, Di Sabatino A, Giuffrida P, Fornasa G, Tsilinigri K, et al. (2016) Abnormal thymic stromal lymphopoietin expression in the duodenal mucosa of patients with coeliac disease. Gut 65: 1670-1680.
- Gauvreau GM, Byrne PMO, Boulet LP, Wang Y, Cockcroft D, et al. (2014) Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med 370: 2102-2110.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)
- Advance Research on Alzheimers and Parkinsons Disease
- International Journal of Diabetes (ISSN: 2644-3031)
- Journal of Psychiatry and Psychology Research (ISSN:2640-6136)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)



