3937
Views & Citations2937
Likes & Shares
Autoantibodies to 5’-nucleotidase 1A (NT5c1A/Mup44) are regarded as a biomarker for sporadic inclusion body myositis (sIBM). Among other diseases, anti-NT5c1A has been reported in Sjögren syndrome (SjS) andin up to 27% of juvenile dermatomyositis (JDM). In this serologic study we determined the sensitivity and specificity of anti-NT5c1A in a multi-center, international JDM cohortand in other pediatric autoimmune rheumatic diseases. Sera from 117 JDM patients from Canada, United States and Mexicoas well as controls and comparators were stored at -80°C until required for analysis. The pediatric comparators included juvenile idiopathic arthritis (JIA) (N=118), systemic lupus erythematosus (pSLE) (N=36), localized scleroderma (pLSC) (N=108), and systemic sclerosis (pSSc) (N=114).IgG antibodies to NT5c1A were detected by an addressable laser bead immunoassay (ALBIA) using a full-length human recombinant proteinas previously published, and a cut-off which was two standard deviations above the mean of pediatric healthy controls. ANA was detected by immunofluorescence assay (IFA) on a HEp-2 substrate.
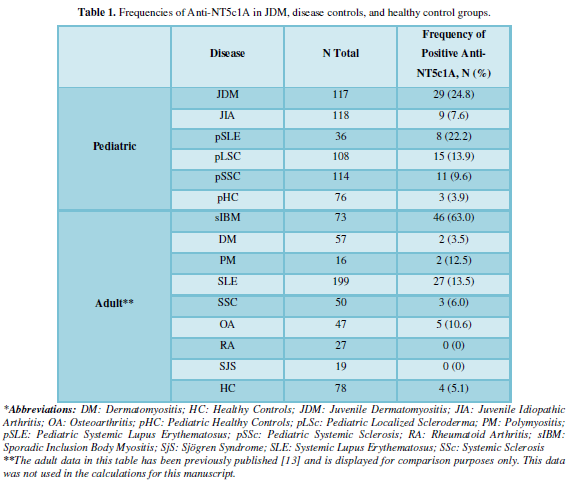
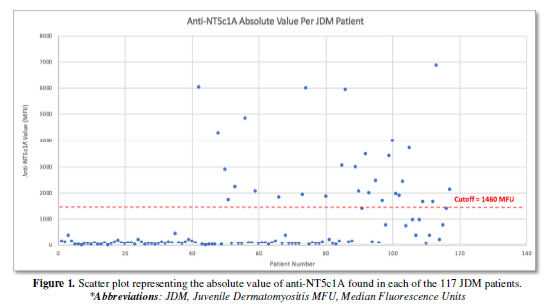
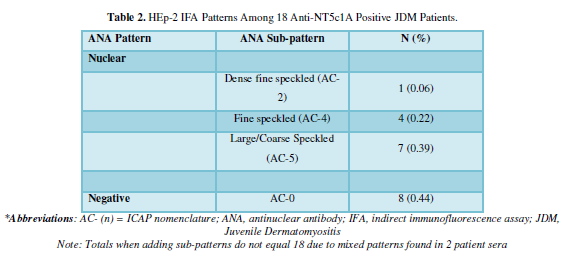
In the expanded JDM cohort, 29/117 (24.8%) were positive for anti-NT5c1A. 43/376 (11.4%) of our pediatric disease comparators were positive for anti-NT5c1A. Specifically, 9/118 (7.6%) with JIA, 8/36 (22.2%) patients with pSLE, 15/108 (13.9%) with pLSc, and 11/114 (9.6%) pSSC were positive for anti-NT5c1A. The frequency in pediatric healthy controls was 3/76 (3.9%). The sensitivity, specificity, positive and negative predictive values of anti-NT5c1A in JDM were 0.25 [95% CI: 0.17, 0.33], 0.90 [95% CI: 0.87, 0.93], 0.39 [95% CI: 0.28, 0.50] and 0.82 [95% CI: 0.79, 0.86] respectively among our pediatric patients. In 18 JDM patients who were positive for anti-NT5c1A, the ANA indirect immunofluorescence assay (IFA) on HEp-2 substrate did not demonstrate a consistent staining pattern.
In summary, the frequency of anti-NT5c1A in JDM was 24.8% in our international multi-centre JDM cohort. Although anti-NT5c1A antibodies are relatively common in JDM compared to other connective tissue diseases, they have a low sensitivity but a relatively high specificity for JDM among pediatric patients. This is the first study to report anti-NT5c1A in patients with pSLE, pLSc, and pSSc. Screening for anti-NT5c1A antibodies using HEp-2 IFA is unlikely to be useful in detecting these autoantibodies.Keywords: Anti-NT5c1A, Autoantibodies, Juvenile Dermatomyositis, Biomarker, Pediatric systemic autoimmune rheumatic diseases
Abbreviations: AC: Anti-cell pattern (ICAP); ALBIA: Addressable laser bead immunoassay; CI: Confidence interval; DM: Dermatomyositis; DNA: Deoxyribonucleic acid; ICAP: International consensus on autoantibody patterns; IFA: Indirect immunofluorescence assay; JIA: Juvenile idiopathic arthritis; MFU: Median fluorescence units; NT5c1A: 5’-nucleotidase 1A (Mup44); OA: Osteoarthritis; OR: Odds ratio; pSLE: Pediatric SLE; pSSc: Pediatric systemic sclerosis; pLSc: Pediatric localized scleroderma; PM: Polymyositis; RA: Rheumatoid arthritis; RNA: Ribonucleic acid; RNP: Ribonucleoprotein; SjS: Sjögren’s syndrome; sIBM: Sporadic inclusion body myositis
METHODS
Patients and controls
This research was approved by the Health Research Ethics Boards (HREB) at each centre and performed in accordance with the Helsinki Declaration of 1975 as revised in 2013. When needed, written informed consent was acquired.
Autoantibody testing
RESULTS
DISCUSSION
In addition, this is the first study to report frequency of anti-NT5c1A in pSLE, pLSc and pSSc. Most notably, the frequency in pSLE was 22.2% (8/36), which is close to the frequency in JDM. sIBM has been reported in SLE [23,24] but not pSLE, and 17% of pSLE were reported to have myositis, some of which were associated with anti-Ribonucleoprotein (RNP) autoantibodies [25]. In adult SLE, the frequency of anti-NT5c1A was reported to be as high as 20% [26]. In this study of adult SLE, there was no relationship between anti-NT5c1A and other autoantibodies, the frequency of muscular symptoms or viral infections but there was a higher frequency of co-morbidity from other autoimmune diseases. Further studies in pSLE patients will be needed to elucidate the clinical and serological correlates of anti-NT5c1A in this population.
The frequency of anti-NT5c1A autoantibodies in a previous study by Yeker et al. [14] of 307 JDM was reported to be 27%. The same study found a frequency of 35% (N=46) in patients with juvenile overlap myositis, and 27% (N=30) in patients with JIA. As comparators in the study, 11% (N=27) of patients with juvenile polymyositis and 12% of healthy control children were positive for anti-NT5c1A [14]. Following the expansion of our JDM cohort, the frequency of 24.8% (N=117) is consistent with this previous report by Yeker et al. In contrast, the frequency among our JIA cohort was lower in comparison to Yeker et al. (7.6% vs 27%), which may have been due to our larger patient cohort or differences in assays used. Yeker et al. also found that children who were anti-NT5c1A autoantibody positive had greater pulmonary symptoms at diagnosis, more frequent hospitalizations, and needed more medications to control their disease [14]. Further studies correlating anti-NT5c1A positivity to clinical and demographic features in pediatric patients with rheumatologic and connective tissue diseases are needed to further explore these relationships, and to ultimately determine the pathogenicity of anti-NT5c1A autoantibodies. Nevertheless, it is becoming apparent that while anti-NT5c1A autoantibodies are an important biomarker in sIBM, they are also found in a significant proportion of other autoinflammatory rheumatic diseases, including those in children. Since NT5c1A dephosphorylates the 5' and 2'(3')-phosphates of deoxyribonucleotides [27], it is interesting to consider that these autoantibodies belong to a larger family of RNA and DNA macromolecular complexes (i.e. small nuclear RNP, small cytoplasmic RNP, and tRNA synthetases) that are well-known targets in pediatric and adult systemic autoimmune rheumatic diseases [28].
Our repeat HEp-2 IFA analysis of anti-NT5c1A positive JDM sera did not reveal a specific IFA pattern that could be used as a screening test. NT5c1A has been reported to be localized in the cytosol [27], thus it is likely that the mixed IFA findings in our study reflect undetected and confounding antibodies. Future analyses using “monospecific” anti-NT5c1A sera may assist in determining whether a reliable screening ANA IIF pattern exists for anti-NT5c1A autoantibodies.
Limitations of our study include the small sample size of JDM, disease comparators and healthy control patient cohorts. This can be addressed with international multi-centre studies using a full-length anti-NT5c1A protein for detection of autoantibodies, in an ALBIA or equivalent immunoassay, as done here and previously [13]. Unfortunately, no “gold standard” assay exists for anti-NT5c1A autoantibodies, although we found high correlation of our ALBIA with a commercially available enzyme-linked immunosorbent assay (ELISA) when the assays cut-offs were adjusted according to pediatric control samples. This was a cross-sectional study and, therefore, we were unable to assess if anti-NT5c1A antibodies were present at the time of diagnosis and/or if they remained stable over time. Last, we did not study clinical or demographic correlations in our pediatric cohorts because the serum samples were drawn from seven geographically dispersed centers where a unified ethics protocol and appropriate patient consent presented challenges. Hence, we were unable to study comorbidities such as myopathy or other features of sIBM. Given the presence of anti-NT5c1A antibodies in diverse pediatric autoinflammatory conditions, it seems unlikely that features of sIBM would be found. However, there is a possibility that a unique clinical phenotype may emerge from prospective longitudinal studies.
In summary, we have found that anti-NT5c1A has a poor sensitivity but a relatively high specificity for JDM. This study is also the first to report frequencies of anti-NT5c1A in pSLE, pLSc, and pSSc. Furthermore, we were unable to identify a consistent HEp-2 IFA staining pattern among anti-NT5c1A positive JDM patients, thus ANA test is not a useful screening test for detection of these autoantibodies. Additional studies are needed to further delineate pathophysiologic mechanisms and the clinical, demographic, and serological associations of anti-NT5c1A antibodies among patients with inflammatory myositis.
-
Bianchi V, Spychala J (2003) Mammalian 5’-nucleotidases. J Biol Chem 278(47): 46195-46198.
-
Kviklyte S, Vertommen D, Yerna X, Andersén H, Xu X, et al. (2017) Effects of genetic deletion of soluble 5′-nucleotidases NT5C1A and NT5C2 on AMPK activation and nucleotide levels in contracting mouse skeletal muscles. The American J Phys-Endocrinology and Metabolism 313(1): 48-–62.
-
Hunsucker SA, Mitchell BS, Spychala J (2005) The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacology and Therapeutics 107(1): 1-–30.
-
Pluk H, van Hoeve BJ, van Dooren SH, et al. (2013) Autoantibodies to cytosolic 5'-nucleotidase 1A in inclusion body myositis. Ann Neuro 73: 397-407.
-
Salajegheh M, Lam T, Greenberg SA (2011) Autoantibodies against a 43 KDa Muscle Protein in Inclusion Body Myositis. PLoS ONE 6: e20266.
-
Larman HB, Salajegheh M, Nazareno R, et al. (2013) Cytosolic 5'-nucleotidase 1A autoimmunity in sporadic inclusion body myositis. Ann Neuro 73: 408-418.
-
Lloyd TE, Christopher-Stine L, Pinal-Fernandez I, et al. (2015) Cytosolic 5'-nucleotidase 1A is a common target of circulating autoantibodies in several autoimmune diseases. Arthritis Care & Research (Hoboken) 68: 66-71.
-
Goyal NA, Cash TM, Alam U, et al. (2016) Seropositivity for NT5c1A antibody in sporadic inclusion body myositispredicts more severe motor, bulbar and respiratory involvement. J Neuro Neurosurg and Psy 87: 373-–378.
-
Herbert MK, Stammen-Vogelzangs J, Verbeek MM, et al. (2016) Disease specificity of autoantibodies to cytosolic 5'-nucleotidase 1A in sporadic inclusion body myositis versus known autoimmune diseases. Ann Rheum Dis 75(4) : 696-701.
-
Lilleker JB, Rietveld A, Pye SR, et al. (2017) Cytosolic 5'-nucleotidase 1A autoantibody profile and clinical characteristics in inclusion body myositis. Annalals of the Rheumatic Disseases 76: 862-868.
-
Tawara N, Yamashita S, Zhang X, et al. (2017) Pathomechanisms of anti-cytosolic 5'-nucleotidase 1A autoantibodies in sporadic inclusion body myositis. Annalls of Neurology 81: 512-525.
-
Muro Y, Nakanishi H, Katsuno M, et al. (2017) Prevalence of anti-NT5C1A antibodies in Japanese patients with autoimmune rheumatic diseases in comparison with other patient cohorts. ClinicaChimica Acta 472: 1-4.
-
Amlani A, Choi MY, Tarnopolsky M, et al. (2019) Anti-NT5c1A autoantibodies as biomarkers in inclusion body myositis. Frontiers in Immunollogy 10: 745.
-
Yeker RM, Pinal-Fernandez I, Kishi T, et al. (2018) Anti-NT5C1A autoantibodies are associated with more severe disease in patients with juvenile myositis. Annals of the Rheumatic Diseases 77: 714-719.
-
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (second of two parts). New England J Journal of Med icine 292: 403-–417.
-
Eystathioy T, Chan EK, Takeuchi K, et al. (2003) Clinical and serological associations of autoantibodies to GW bodies and a novel cytoplasmic autoantigen GW182. Journal of Mollecular Medicine 81: 811-–818.
-
Selak S, Mahler M, Miyachi K, et al. (2003) Identification of the B-cell epitopes of the early endosome antigen 1 (EEA1). Clinical Immunology 109: 154-1–64.
-
Copple SS, Jaskowski TD, Giles R, et al. (2014) Interpretation of ANA indirect immunofluorescence test outside the darkroom using NOVA view compared to manual microscopy. J Immun Res 2014: 149316.
-
Chan EK, Damoiseaux J, Carballo OG, et al.(2015) Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014-2015. Frontiers in Immunology 6: 412.
-
Greenberg, SA (2019) Inclusion body myositis: Cclinical features and pathogenesis. Nature Reviews Rheumatology 15(5): 257-272.
-
Yasin SA, Schultz PW, Deakin CT, et al. (2019) Histological heterogeneity in a large clinical cohort of juvenile idiopathic inflammatory myopathy: Aanalysis by myositis autoantibody and pathological features. Neuropathol Appl Neurobiol 45: 495-512.
-
Deakin CT, Yasin SA, Simou S, et al. (2016) Muscle biopsy in combination with myositis-specific autoantibodies aids prediction of outcomes in juvenile dermatomyositis. Arthritis Rheumatol 68(11): 2806-–2816.
-
Limaye V, Scott G, Kwiatek R, Pile K (2000) Inclusion body myositis associated with systemic lupus erythematosus (SLE). Aust NZJ Med 30: 275-276.
-
Derk CT, Vivino FB, Kenyon L, Mandel S (2003) Inclusion body myositis in connective tissue disorders: Ccase report and review of the literature. Clin Rheumatol 22: 324-328.
-
Record JL, Beukelman T, Cron RQ (2011) High prevalence of myositis in a southeastern United States pediatric systemic lupus erythematosus cohort. PediatrRheumatol Online J 9: 20-29.
-
Rietveld A, van den Hoogen LL, Bizzaro N, et al. (2018) Autoantibodies to Cytosolic 5'-Nucleotidase 1A in Primary Sjogren's Syndrome and Systemic Lupus Erythematosus. Front Immunol 9: 1200.
-
The UniProt Consortium (2019) UniProt: Aa worldwide hub of protein knowledge. Nucleic Acids Research 47: D506-515.
-
Satoh M, Fritzler MJ, Chan EKL (2020) Antihistone and Antispliceosome Antibodies. In: Lupus Erythematosus: Basic, Applied and Clinical Aspects. , edited by G. C. Tsokos, New York: Academic Press, pp: 237-247.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Journal of Infectious Diseases and Research (ISSN: 2688-6537)
- Journal of Allergy Research (ISSN:2642-326X)
- International Journal of Internal Medicine and Geriatrics (ISSN: 2689-7687)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Neurosurgery Imaging and Techniques (ISSN:2473-1943)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)



